Introduction
Many facilities have chemically reactive materials and systems without knowing the hazards they pose. Others are aware of the hazards, but have inadequate safeguards. Still others have situations where materials are adequately controlled individually, but the potential for a major incident exists if materials are inadvertently combined. Here are five examples:
- Rainwater leaked into a room where hundreds of drums of dry swimming pool chemicals were stored, causing an explosion. The explosion and resulting fire set off the sprinkler system that soaked the remaining drums. The fire, explosions and chlorine releases lasted three days. Over 25,000 people were evacuated, and 275 people went to the hospital with skin burns and respiratory problems.
- Twenty-three people were sent to the hospital after a chemical release at a resort casino. Two cleaning agents were apparently mixed together in the basement of the building, generating vapors that permeated part of the resort.
- A runaway reaction and reactor explosion occurred in a resins production facility that killed one worker and injured four others. To control the reaction rate, an operating procedure called for the slow addition of one of the raw materials to the reactor. The runaway was triggered when the raw materials and catalysts were improperly charged to the reactor simultaneously, followed by heat addition.
- A massive explosion and fire occurred at an agricultural chemical packaging facility in Arkansas, killing three firefighters and injuring a fourth. The likely cause was a supersack of azinphos-methyl (an insecticide) being placed near a hot compressor exhaust pipe.
- Five workers were killed when a blender exploded. The blender was used to mix several dry powders, including aluminum powder and sodium hydrosulfite. The likely cause of the explosion was the unintentional introduction of water into the blender, possibly through a leaking water-cooled seal.
This document is intended as an introduction to reactive material issues for people whose main business is not reactive materials and systems. Further, it does not replace any of the more extensive guides and references that deal with this topic in detail, or eliminate the need for competent expert analysis in dealing with these issues. The last section of this document lists references and sources of information that readers are strongly encouraged to use.
Reactivity is the tendency of a material or combination of materials to undergo chemical change under the right conditions. Chemical reactivity is a highly desirable trait that permits a wide variety of useful materials to be synthesized. It also allows products to be made under relatively moderate conditions of pressure and temperature, saving energy and reducing the physical risks of hightemperature and/or high-pressure equipment. Even some consumer products such as swimming pool chemicals have reactive properties. On the other hand, it is these properties that make reactive materials so useful that also pose hazards to health and property, and reactions are not limited to intended and controlled situations.
You may find it helpful to identify and control reactivity hazards in two broad categories: reactive materials and reactive interactions. Reactive materials are commonly regarded as those materials that can be hazardous by themselves when caused to react by heat, pressure, shock, friction, a catalyst, or by contact with air or water. Reactive interactions require the combining of two or more materials to pose a hazardous situation by chemical reaction.
So, what do you need to know about reactive materials and interactions? The following diagram will guide you through the four key questions that you must be able to answer. The best time to consider these questions is when designing a new operation or facility, but they should be answered for existing operations as well, particularly when making changes or bringing in new materials.
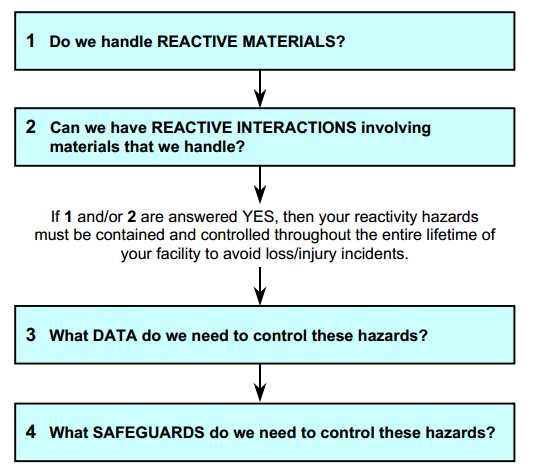
Read more
- Do we handle Reactive Materials?
- Can we have Reactive Interactions?
- What data do we need to control these hazards?
- What safeguards do we need to control these hazards?
- Where Can We Get More Help?
A final word, quoted from The Dow Chemical Company, a leader in the field of reactive chemistry:
"We recognized long ago that virtually any chemical can be reactive if involved in the wrong situation or scenario. We therefore do not limit our hazard assessments to any specific list of chemicals. Some companies limit the scope of their reactive chemicals hazard assessments to scenarios that involve only inadvertent mixing of chemicals. While this type of scenario is an important part of any reactive chemicals program, it is far from all of what needs to be considered in a total reactive chemicals hazard assessment effort. Companies whose work is just chemical handling may find it appropriate to only
address inadvertent mixing, but additional dimensions need to be included for companies involved with processing or reacting chemicals. Some additional types of scenarios beyond inadvertent mixing of chemicals that need to be included in a comprehensive Reactive Chemicals program include:
- Reactor loss of control scenarios and lines of defense
- Inadvertent lack of mixing of things like reaction inhibitors in reactors or storage tanks
- Accelerated corrosion and loss of containment due to material incompatibility
- Special scenarios that result in loss of stability of chemicals"
Acknowledgements
The Center for Chemical Process Safety would like to especially thank Peter N. Lodal, Senior Technical Associate with Eastman Chemical Company in Kingsport, Tennessee, for drafting this document and incorporating comments. Thanks are also extended to the following individuals for their invaluable input during the review phase of this document, and to their companies for making them available: Gary Phillips and Tim Overton of The Dow Chemical Company; Donald Connolley of AKZO Nobel Chemicals, Inc.; Robert Stankovich of Eli Lilly and Company; Anthony Thompson of Monsanto Company; and Gregory Keeports and Dennis Hendershot of Rohm and Haas Company. Jack Weaver, Lester Wittenberg, Scott Berger, and John Bresland provided CCPS input, support, and project coordination. Robert W. Johnson, Principal Consultant with Unwin Company in Columbus, Ohio, contributed technical content and provided technical review and editing support.
