No disease-modifying drug exists for osteoarthritis due to poor drug delivery within joints. Engineered biomaterials could address this challenge by improving the duration and targeting of therapies.
Osteoarthritis is a debilitating disease of individual joints, marked by progressive joint tissue degeneration, which causes pain and loss of mobility. It is a widespread disease that affects 30 million people in the U.S., including 19% of adults aged 45 and older (1). However, despite decades of research and development, no disease-modifying drug for osteoarthritis has been approved for use in humans (2). Such a drug could slow disease progression by reducing the rate of cartilage degeneration or even regenerating new tissue. The current standard of care focuses on pain relief only after symptoms are present. Even approved drugs in this category, such as corticosteroids and hyaluronic acid suspensions, are subject to debate with respect to their safety and/or efficacy (3–5).
Underlying the clinical failures of disease-modifying drugs and the shortcomings of approved drugs is inadequate drug delivery to target joint tissues (6, 7). Despite the use of intra-articular injection as a technique for local delivery to the joint, free drugs are unable to remain within the joint space for adequate time periods and thereby do not reach their biological targets at sufficient levels (8).
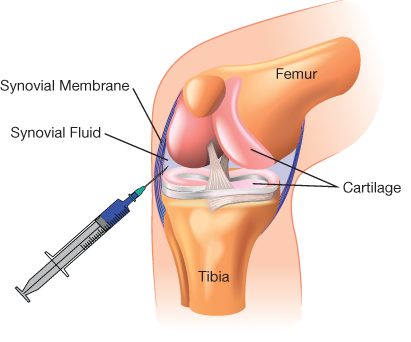
▲Figure 1. Intra-articular injection is commonly used for local drug delivery into knee joints. However, upon injection into the fluid-filled joint capsule encased in the synovial membrane (or synovium), the drug is typically lost to systemic circulation within a matter of hours to days.
The key obstacle for drug delivery in osteoarthritis is the hostile pharmacokinetics of the joint. Upon injection into the articular joint capsule (Figure 1), the drug enters synovial fluid, which is subject to rapid physiological turnover (8). The fluid and the drug contained within it are rapidly drained via the venules and lymphatic vessels located in the synovial membrane; hence, most drugs are lost to systemic circulation (9).
Free drugs are cleared from articular joints in a matter of hours to days, with some dependence on the molecular weight of the drug molecule. In contrast to this short therapeutic time frame, most clinicians seek to minimize the frequency of repeat intra-articular injections. Time between injections varies based on the physician’s judgment and the drug being used, but an interval of 2–12 weeks is considered reasonable. It is therefore unsurprising that many treatments for osteoarthritis are ineffective.
Moreover, articular cartilage, which is often the therapeutic target of disease-modifying drugs, presents a formidable biological barrier to drug delivery. Cartilage is avascular (i.e., it has no blood vessels), and thus penetration of drugs through the tissue to interact with the resident cell type, chondrocytes, occurs only by diffusion through the cartilage. Diffusive transport through cartilage is significantly hindered by its dense, highly anionic extracellular matrix and small pore size of less than 15 nm (10). Diffusion through cartilage is slower than the clearance rate of the joint, so free drug in the joint space is typically cleared before it can penetrate the depth of cartilage at a therapeutic concentration.
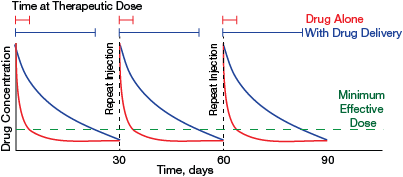
▲Figure 2. Improved drug delivery would extend the residence time of intra-articular drug therapies in joints, which would markedly increase the total time at therapeutic dose over the course of treatment. Clinicians aim to reduce injection frequency as much as possible while still maintaining an effective drug concentration. Advanced drug delivery systems developed by researchers can make this goal possible.
Fortunately, advanced formulation techniques for intra-articular injection using engineered biomaterials show promise in overcoming these delivery challenges. Even modest improvements in intra-articular penetration and half-life could have a considerable impact on therapeutic drug exposure time between injections (Figure 2).
This article provides an overview of some of the design strategies used in drug delivery systems for joints, and discusses important considerations and challenges for clinical translation of these technologies.
Strategies to avoid joint clearance: Microparticles
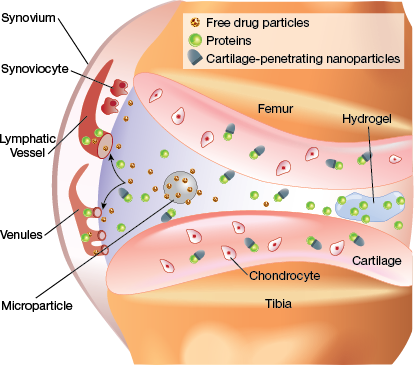
▲Figure 3. In articular joints, free drugs and unbound nanoparticles are quickly cleared into lymphatic vessels and venules in the synovium. Microparticles and hydrogels are retained within the synovial fluid and slowly release drugs until they degrade. Cartilage-penetrating nanocarriers enter the tissue and interact with cells, although those that do not bind to cartilage are cleared.
One approach to prevent clearance of a drug from synovial fluid is to encapsulate the drug in a biomaterial package that is simply too large to enter the synovial microvasculature. The biomaterial, with its longer joint residence time, can serve as a controlled release depot for the drug over a much longer timescale than an injection of a free drug (Figure 3). This tactic can also reduce the maximum concentration of the drug to which joint tissues are exposed. A reduced maximum concentration of drug is particularly important for corticosteroids, which have shown concerning side effects at repeated high doses (5, 11), as well as for potent biologic drugs.
Flexion Therapeutics used this approach in developing FX-006, a therapy that was recently approved by the U.S. Food and Drug Administration (FDA). FX-006 is a poly(lactic-co-glycolic acid) (PLGA) microparticle that encapsulates triamcinolone acetonide (TA), a clinically used corticosteroid that targets synovial tissue to reduce inflammation and pain. The PLGA microparticles have a median size of 42 µm, which is large enough to prevent clearance through joint microvasculature (12).
In humans, TA released from FX-006 was measurable in synovial fluid in most patients through 12 weeks post-injection, whereas TA in crystalline suspension was below the lower limit of quantification by six weeks (13). The greatly improved pharmacokinetics of FX-006 produced statistically significant improvements in joint pain, function, and stiffness that warranted FDA approval of the therapy.
Importantly, the possibility that clinical improvement can be achieved using already approved therapeutics for osteoarthritis pain suggests that further advanced delivery approaches could enable the success of true disease-modifying drugs. A cartilage drug delivery system could sufficiently improve the efficacy of a previously failed disease-modifying drug to show clinical benefit.
Strategies to avoid joint clearance:...
Would you like to access the complete CEP Article?
No problem. You just have to complete the following steps.
You have completed 0 of 2 steps.
-
Log in
You must be logged in to view this content. Log in now.
-
AIChE Membership
You must be an AIChE member to view this article. Join now.
Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
