 The global shortage of transplantable organs has long been recognized as a significant challenge in the organ transplant supply chain. Only 10% of the worldwide need for organ transplantation is being met. On average, only 35% of all organs authorized for transplant are actually transplanted. Among the large solid organs, the lungs, intestine, and heart have the lowest utilization, partially due to their very short preservation times.
The global shortage of transplantable organs has long been recognized as a significant challenge in the organ transplant supply chain. Only 10% of the worldwide need for organ transplantation is being met. On average, only 35% of all organs authorized for transplant are actually transplanted. Among the large solid organs, the lungs, intestine, and heart have the lowest utilization, partially due to their very short preservation times.
Although the U.S. completes about 40,000 transplants each year, a patient passes away every single hour waiting for a lifesaving organ. Many transplantable organs are wasted, especially sensitive organs such as the heart. In fact, 80% of donor thoracic organs are not utilized for transplant. Since the first successful organ transplant more than sixty years ago, the transplant field has experienced significant improvements in surgical techniques and immunosuppression. However, development in the past four decades has seriously lagged in innovations to extend the timeframe of safe cold organ transport.
During transport, the organ can suffer ischemic injury due to lack of blood supply. Preserving tissues and organs at low temperature on wet ice (about 4°C) has thus far been the most effective way to reduce metabolism and prevent injury to the organ. However, this approach crucially limits cold storage times to 4–16 hr depending on the type of organ. Although reducing the temperature further would help extend the life of the organ, a temperature less than 0°C allows ice crystals to form, which causes irreversible freezing damage. One approach could involve submerging the organ in antifreeze cryoprotective agents (CPAs) at very high volume percentages to prevent freezing at low temperatures; however, these agents are toxic to the organ.
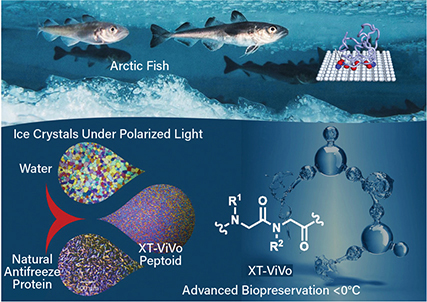
▲ Antifreeze proteins are found in many aquatic and terrestrial species (top). X-Therma used these molecular structures to develop a peptoid (bottom) that can shape ice into rounded structures.
X-Therma, Inc., a National Science Foundation (NSF)-supported startup that began in the User Program of the Molecular Foundry at Lawrence Berkeley National Lab, is developing a breakthrough biopreservation technology to address the critical challenges of organ transport. The company’s first-in-the-field technology was inspired by antifreeze proteins in arctic fish that effectively bind nucleated ice to control ice growth and avoid cellular damage.
The team used drug discovery approaches for the de novo development of new low-molecular-weight protein-mimetic oligomers — namely peptoids — that preserve the important functional attributes of antifreeze proteins and act as highly-effective nontoxic CPAs. From the multiple peptoids the researchers discovered, they chose one candidate for further development. The peptoid can be used at very low concentrations, which addresses the toxicity issues related to classic CPAs.
“This chemistry encodes specific protein-like function into a synthetic molecule to shape ice crystals and prevent them from growing bigger,” says Mark Kline, CTO of X-Therma. In partnership with Johns Hopkins Univ. and other teams, X-Therma demonstrated in a preclinical animal model that a heart can be brought back to life after ice-free subzero preservation and the transplant window can be extended to more than 24 hours.
The company’s first product for cell preservation, a nontoxic and serum-free cryopreservation solution, removes some primary bottlenecks that biopharma companies face when scaling and manufacturing cell therapies such as cancer treatments. The product could also increase reliability in cold-chain logistics.
For next steps, the company is focused on rolling out a Current Good Manufacturing Practice (cGMP)-grade cell preservation product in 2021 for clinical use in cell therapy. “In cell therapy, X-Therma products provide a huge advantage in the manufacturing process, especially in the process of filling and transportation,” comments Steve Oh, Director of Stem Cell Bioprocessing at A*STAR in Singapore.
Over the coming years, X-Therma expects to see a transformative impact on global organ transplant supply. “Removing time from the equation, you can walk into a global organ bank to get a perfectly matched heart to save your life, potent cell therapy to cure cancer, and 3D-printed tissue to repair and rejuvenate your body whenever, wherever you need,” says X-Therma CEO Xiaoxi Wei.
This technology was funded through the NSF Small Business Innovation Research (SBIR) Program.
This article was prepared by the National Science Foundation in partnership with CEP.

Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
