Traditional methods of gene delivery to plants are labor- and time-intensive, are suitable for only a small number of hosts, and have high toxicity and limited practical applicability. This article discusses how nanoparticle-based approaches could enable efficient gene transfer into plants.

▲Figure 1. Plant synthetic biology has many uses and applications across several industries.
Plant synthetic biology has numerous applications in agriculture, as well as in the pharmaceutical and energy industries (Figure 1). In agriculture, genetic engineering of plants can be employed to create crops that are resistant to herbicides, insects, diseases, and drought. The ability to introduce transgenes into plant cells also provides the opportunity to improve the nutrient profile of a crop. In the pharmaceutical industry, genetically engineered plants could be used to synthesize valuable small-molecule drugs. Genetically modified plants could also make biofuel production more efficient, which would provide a major benefit for the energy industry.
A crucial first step of plant genetic engineering, regardless of the application, is to deliver genes into the plant cells. Ever since the first transgenic plants were created in the 1980s, researchers have endeavored to develop and advance new gene delivery systems for plants. This article briefly explains conventional methods of delivering genes to plants and their strengths and limitations. It also describes newer methods of gene delivery that are based on nanoparticle transport, and demonstrates how they may impact the field of plant gene transfer and engineering.
Conventional gene delivery techniques
Conventional methods to deliver genes to plant cells can be grouped into three categories: physical, chemical, or biological approaches (Table 1). The most common and preferred physical gene delivery methods are biolistic particle delivery (also called particle bombardment or gene gun delivery) and electroporation (the use of electric field pulses to create pores in cell membranes). Chemical delivery methods use polymers and cationic lipids as transfer agents. For example, polyethylene glycol (PEG)-mediated delivery is one of the frequently used chemical delivery methods.
| Table1. Conventional gene delivery methods can be grouped into physical, chemical, and biological categories. Each has its own strengths and limitations. | |||
| Conventional Gene Delivery Methods | Strengths | Limitations | |
| Physical | Biolistic Particle Delivery |
|
|
| Electroporation |
|
| |
| Chemical | PEG-Mediated Delivery |
|
|
| Biological | Agrobacterium-Mediated Delivery |
|
|
Among the three approaches, however, biological methods are favored over physical and chemical methods, because they have higher transformation efficiencies in plant systems. Gene delivery via Agrobacterium is frequently used in plant genome engineering.
Biolistic particle delivery
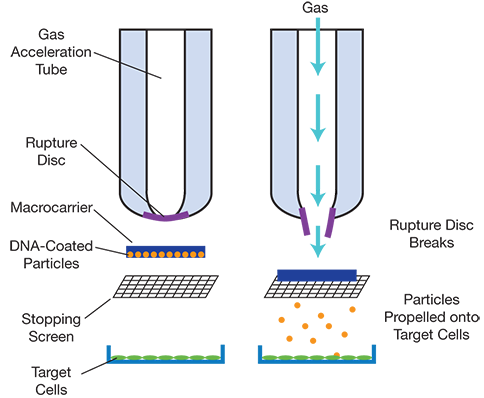
▲Figure 2. A gene gun, or biolistic particle delivery device, can deliver transgenes to cells. First, a macrocarrier is loaded with DNA-coated heavy-metal particles. Within the gene gun, gas pressure builds up against a rupture disk. The pressure eventually reaches a point that causes the rupture disc to break, and the pressure burst propels the macrocarrier into a stopping screen. The DNA-coated particles are propelled through the screen and hit the target cells.
Biolistic particle delivery was developed in 1982 by Sanford, et al.(1). In biolistic delivery, genes are coated and dehydrated onto heavy-metal particles, such as gold or tungsten. High-pressure helium pulses accelerate the particles, propelling them into plant cells at high velocities (Figure 2). Typically, the epidermal tissue of plant cells is targeted. Depending on the experimental parameters, DNA can pass through both the plant cell wall and plasma membrane, and can also penetrate into the nucleus (2). Helium gas pressure, net particle size, and dosing frequency are critical experimental parameters that determine the penetration efficiency, toxicity, and overall gene transfer levels in plants.
Biolistic particle delivery facilitates gene delivery and genome editing by transferring thousands of DNA molecules of sizes up to 150 kilobase (kb) (3). Although the method is inexpensive and easy to perform, researchers have concerns about the integrity of the DNA after transfer, the short-term and low-level expression of the delivered genes in the plants, and cell damage from the high pressures that plant tissues experience.
Electroporation
In electroporation, strong electric field pulses alter the cell’s permeability and generate transient pores in the cell membrane that allow the transport of genes into the plant cell cytoplasm.
Electroporation was developed in vitro for proto-plast (i.e., plant cells with enzymatically degraded cell walls) transformation in 1982 (4). However, electroporation has since been shown to successfully initiate transfection (i.e., inserting genetic material into cells) within intact plant cells, in vivo, as well...
Would you like to access the complete CEP Article?
No problem. You just have to complete the following steps.
You have completed 0 of 2 steps.
-
Log in
You must be logged in to view this content. Log in now.
-
AIChE Membership
You must be an AIChE member to view this article. Join now.
Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
