Sections
Ethylene is a simple yet powerful molecule that serves as the foundation for plastics, chemicals, and everyday products, making it one of the global economy’s most essential building blocks.

▲ Nighttime view of the Borouge 2 Ethylene Plant in the United Arab Emirates. Image courtesy of Linde Engineering.
Ethylene is the unsung hero of the modern chemical industry, serving as the foundation for a vast range of products that improve daily life. As a key petrochemical, ethylene is used to manufacture essential materials, including plastics, packaging, textiles, detergents, and medical supplies (1–4). Its simple molecular structure makes it highly versatile, allowing it to be transformed into polymers and chemicals that support industries ranging from healthcare and transportation to construction and consumer goods. Without ethylene, many of the products we rely on for convenience, safety, and sustainability would not exist.
One of the most significant applications of ethylene is in the production of plastics, which have become indispensable due to their durability, lightweight nature, and ability to be engineered for specific functions. Polyethylene, the most widely used plastic, is derived from ethylene and is utilized in a diverse range of applications, including food packaging, water pipes, medical equipment, and high-performance materials used in the aerospace industry. Figure 1 illustrates this value chain, beginning with ethylene as a foundational building block and culminating in the end products we encounter daily (1).
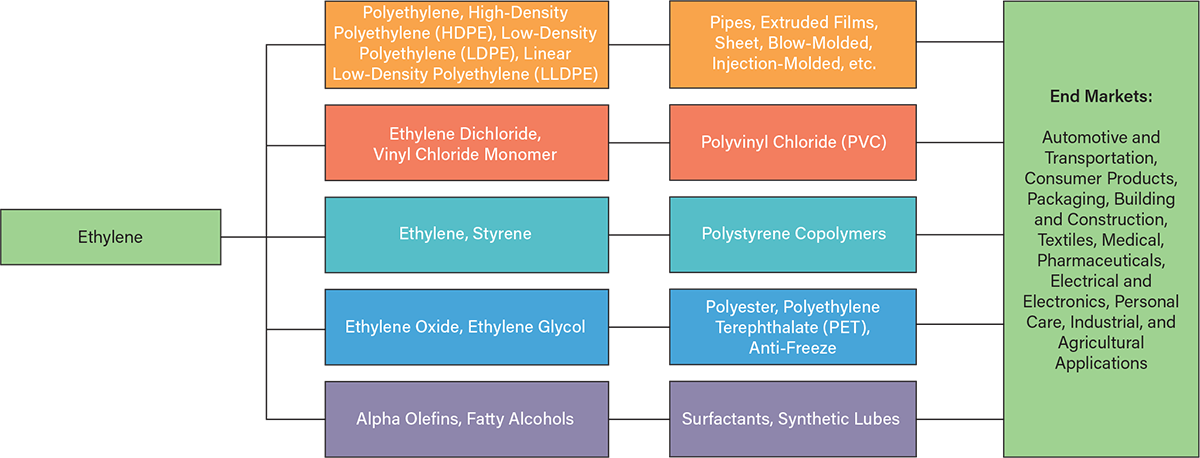
▲Figure 1. Ethylene is converted into key chemical intermediates such as polyethylene, vinyl chloride, styrene, and ethylene glycol, which are then processed into widely used materials and products. These products serve a broad range of end markets, such as the automotive, consumer products, and electronics industries (1).
Plastics help improve energy efficiency, reduce material waste, and enhance product longevity, making them a critical part of modern infrastructure and technology. Advances in plastic recycling and the development of biobased alternatives further improve the sustainability of ethylene-based materials, ensuring their continued role in shaping a more efficient and environmentally responsible future.
As industries evolve and the demand for high-performance, sustainable materials grows, ethylene will remain at the heart of innovation. It is crucial in developing eco-friendly packaging, lightweight automotive parts that improve fuel efficiency, and advanced medical materials that enhance patient care. With continuous improvements in production efficiency, circular economy initiatives, and the introduction of new technologies such as carbon capture and bioethanol-based ethylene, this essential molecule will continue to drive progress across various industries. Ethylene is more than just a chemical; it’s a key enabler of modern life, shaping how we live and work.
Introduction to ethylene
Ethylene (C2H4) is a simple yet highly reactive hydrocarbon molecule consisting of two carbon atoms connected by a double bond. This double bond makes ethylene highly versatile, serving as a key precursor in producing plastics, chemicals, and other essential materials. Naturally occurring in plants as a hormone that regulates growth and ripening, ethylene has also become a key component of the modern petrochemical industry.
The industrial production of ethylene began in the late nineteenth century through the dehydration of ethanol and as a byproduct of coke oven gas production (1–4). In the early 20th century, ethylene was produced from the cracking of ethane, and later, from the cracking of liquid hydrocarbon feeds. Its significance grew with the rise of the plastics industry in the 1930s and 1940s, when large-scale cracking processes were developed to produce ethylene using steam cracking of hydrocarbons. The post-World War II era saw rapid expansion as ethylene-based plastics, such as polyethylene, revolutionized packaging, construction, and consumer goods. The global ethylene capacity grew from nearly 1.5 million metric tons (m.t.) per annum in the mid-1950s to current levels of 225 million m.t. per annum. During the same period, the capacity of a single world-scale unit has increased from 70,000 m.t./yr to more than 2 million m.t./yr.
Over time, technological advancements have improved the efficiency of ethylene production and increased its capacity. Today, ethylene is the world’s most widely produced organic chemical, essential to industries ranging from automotive and healthcare to energy and electronics. It continually evolves to meet growing demands while driving innovation in sustainability and materials science.
Steam cracking chemistry
Steam cracking — the primary industrial process of producing ethylene and other valuable chemicals — involves breaking down larger hydrocarbon molecules (2, 3, 5). This process occurs at high temperatures (750–900°C or 1,380–1,650°F) in the presence of steam, which prevents unwanted coke formation and helps control the reaction. The cracking process follows a free radical mechanism, where heat energy breaks chemical bonds, creating highly reactive free radicals that drive the formation of smaller molecules. Free radical chemistry involves three basic steps.
Step 1: Initiation. At high temperatures, the C–C bonds in feedstocks like ethane, propane, and naphtha break, forming free radicals — highly unstable molecules with unpaired electrons. For example, when ethane (C2H6) is cracked, the reaction creates an ethyl radical (C2H5•) and a hydrogen radical (H•):
C2H6 → C2H5• + H•
These highly reactive radicals will further break down into smaller molecules or recombine. This step is typically the rate-controlling step in steam-cracking furnaces.
Step 2: Propagation. Once free radicals form, they undergo further reactions, forming additional radicals to propagate the reaction chemistry. This step employs various reaction types, including hydrogen abstraction, radical addition, radical decomposition, and radical isomerization, to further advance the cracking process. This step can result in the formation of olefins, such as ethylene (C2H4), propylene (C3H6), and butadiene (C4H6).
For example, the ethyl radical (C2H5•) can lose a hydrogen atom to form ethylene:
C2H5• → C2H4 + H•
Similarly, larger hydrocarbons break down stepwise, forming propylene (C3H6), butadiene (C4H6), and other valuable olefins.
Step 3: Termination. As radicals react with each other or recombine, they reverse the initiation reactions and can form a variety of byproducts. For example:
2 C2H5• → nC4H10
In steam cracking, secondary reactions occur after the initial breakdown through free radical mechanisms. These reactions typically involve smaller molecules reacting to form heavier compounds, such as aromatics, polynuclear aromatics (PNAs), and components heavier than the feed. Olefins and dienes can undergo cyclization to form compounds such as benzene, toluene, and xylenes (Figure 2).
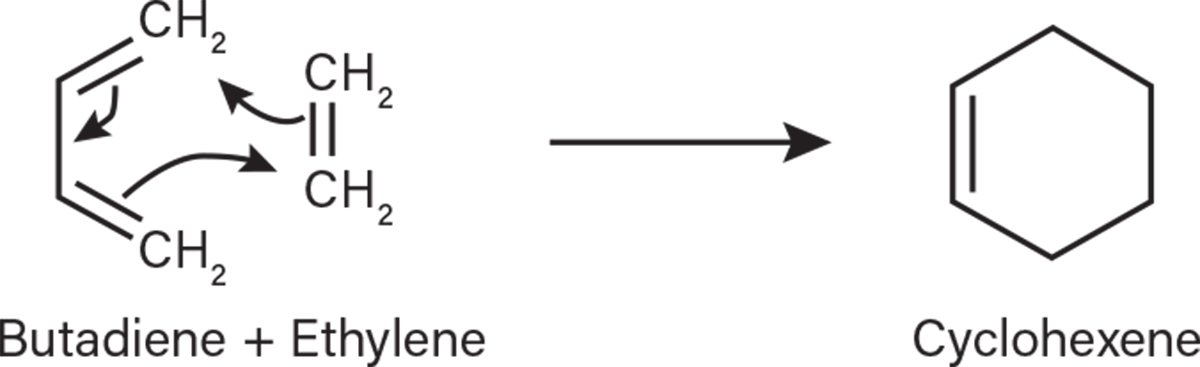
▲Figure 2. Here, butadiene and ethylene undergo cyclization to form cyclohexene.
Further reactions, including dehydrogenation, can lead to the formation of compounds such as naphthalene and anthracene, which are PNAs. PNAs and heavier diolefins can polymerize and dehydrogenate to form coke. High temperatures and longer residence times favor these reactions, resulting in unwanted carbon buildup inside the furnace tubes. Steam is added as a diluent to lower the partial pressure of the hydrocarbons, thereby minimizing side reactions that lead to coke formation. Coke accumulates on the radiant tubes, and tube metal temperatures eventually reach the design metallurgy limits or pressure drop limits. Therefore, the decoking (controlled coke burn) operation is required to bring the furnace back to clean conditions.
Cracking reactions are endothermic (absorbing heat from their surroundings), high-temperature reactions, requiring a heat supply through fuel gas combustion outside the radiant tubes.
The term severity (or conversion) is a measure of the disappearance of feed in the cracking furnace. A higher radiant tube outlet temperature — commonly referred to as coil outlet temperature (COT) — results in a higher severity, producing more olefins in the effluent on a once-through basis. Selectivity measures the amount of olefins (ethylene) produced per feed unit at constant severity. Reactor residence time and hydrocarbon partial pressure determine the selectivity. By controlling reaction conditions, manufacturers optimize yield while minimizing unwanted byproducts, making steam cracking one of the most essential and versatile processes in modern petrochemicals.
Byproducts and their uses. Steam cracking is highly valuable because it produces a range of functional co-products or byproducts (1, 4). Figure 3 illustrates the product distribution for various hydrocarbon feedstocks (2, 3). Some of the co-products and byproducts include:
- Propylene (C3H6): used in the production of polypropylene plastics, automotive parts, and textiles. Like ethylene, propylene is the second most widely produced organic base chemical globally, serving as a key building block for the chemical industry.
- Butadiene (C4H6): used in the production of synthetic rubber for tires and elastomers.
- Aromatics, such as benzene, toluene, and xylene isomers (BTX): used in the production of solvents, fibers, foams, and advanced materials.
- Methane and hydrogen: used as fuel gases or feedstocks for other processes.
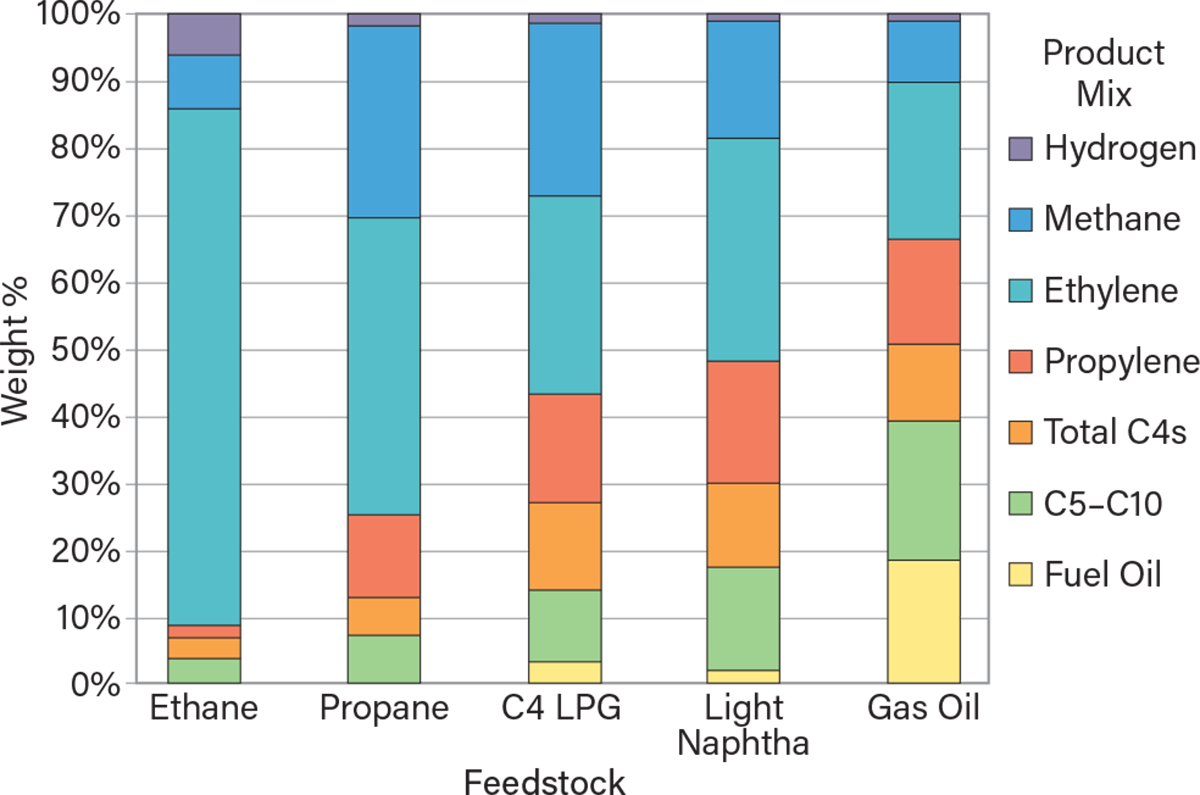
▲Figure 3. Depending on the feedstock, the steam cracker will produce a different mix of byproducts.
Manufacturing process
Figure 4 illustrates the primary processing steps involved in the manufacture of ethylene (1–4). Creating ethylene begins with selecting the right feedstock, depending on geographical location and access to hydrocarbon resources. These feedstocks are hydrocarbons, such as ethane, propane, butane, and naphtha, derived from the extraction of oil and gas. Plant operators carefully choose the optimal mix based on availability and cost-effectiveness. Before entering the process, these hydrocarbons are preheated using heat recovered from other parts of the plant, improving overall efficiency.
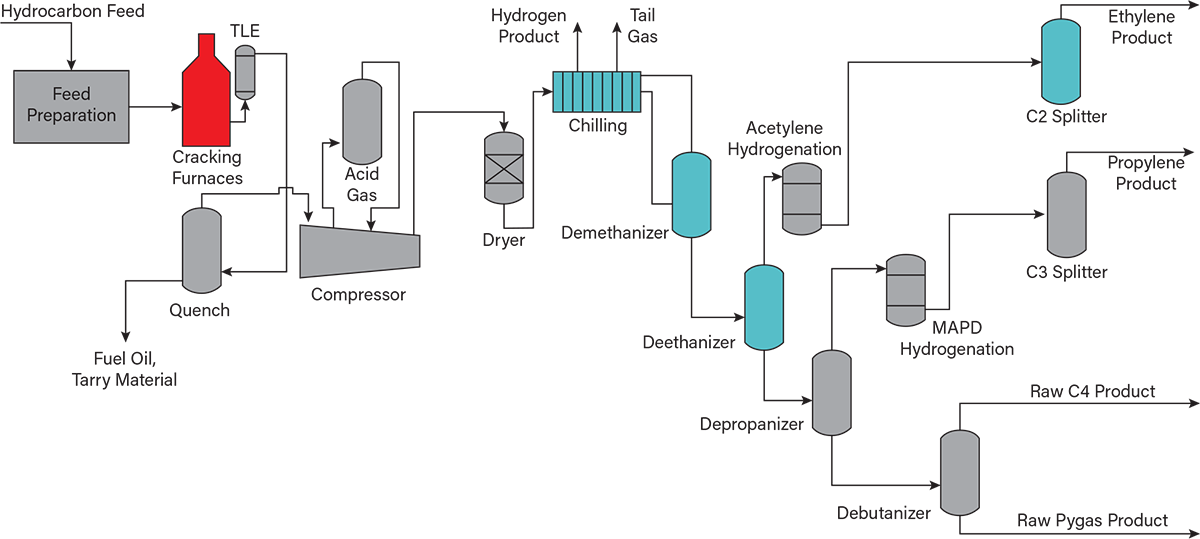
▲Figure 4. This simplified flow diagram illustrates the main steps of ethylene production via steam cracking of hydrocarbon feedstocks. Key process units include cracking furnaces, primary fractionation, and separation trains for recovering ethylene and valuable byproducts like propylene, butadiene (in raw C4s), and pyrolysis gasoline (pygas).
Next, the preheated feedstock is mixed with steam and fed into the steam-cracking furnace — the heart of the operation (Figure 5). The furnace operates between 750°C and 900°C (1,380°F to 1,650°F), breaking down large hydrocarbon molecules into smaller, more valuable ones, such as ethylene and propylene. As discussed earlier, steam helps prevent carbon deposits (coke) from forming on furnace walls and facilitates smooth cracking reactions.

▲Figure 5. At the heart of the ethylene production process are the steam-cracking furnaces. Here, seven cracking furnaces are shown at the Borouge 2 Ethylene Plant in the United Arab Emirates. Photo courtesy of Linde Engineering.
The cracked gas mixture is rapidly cooled in a transfer line exchanger (TLE) to stop unwanted side reactions, quenching the temperature to 350–450°C (660–840°F). The heat recovered in the TLE is utilized for steam generation, which is required for many applications across the plant.
The cooled gas mixture enters the quench section — which typically consists of an oil and water quench for liquid feeds or a water quench for gas feeds — where heavier byproducts, such as fuel oil and tar, are removed. The lighter, more valuable components move forward after cooling and condensation, resulting in a cracked gas stream free of heavy materials and dilution steam.
The purified gas mixture undergoes multi-stage compression, making it easier to handle and process. Acid gases such as hydrogen sulfide (H2S) and carbon dioxide (CO2) are removed using an acid gas scrubber. Subsequently, the cleaned gases are dried before the low-temperature separation steps to prevent the formation of ice and hydrates.
A series of separation towers refines the cracked gas mixture further. These towers include:
- Demethanizer: separates methane and hydrogen from heavies, including ethylene and ethane.
- Deethanizer: separates ethane and lighter gases from heavier components, including propylene and propane.
- Depropanizer: separates propane and propylene from butanes, butadiene, and aromatics (pyrolysis gasoline).
- Debutanizer: separates butanes and butadiene from heavier components, including aromatics (pyrolysis gasoline).
The separation sequence is optimized, in combination with process integration, to achieve overall performance targets.
Refrigeration systems, such as those utilizing propylene, ethylene, and methane cycles, help achieve the low temperatures necessary for effective separation. Mechanical refrigeration systems, which utilize vapor compression and expansion (isenthalpic processes), provide the majority of refrigeration. Depending on the process configuration, isentropic expansion (using turboexpanders) is deployed to achieve the coldest refrigeration level. These systems typically cover the refrigeration needs from ambient temperature conditions down to approximately –140°C (–220°F).
Acetylene and methylacetylene-propadiene (MAPD) are undesirable byproducts that must be removed to ensure that high-purity ethylene and propylene are produced. Selective hydrogenation reactors convert these impurities into more valuable compounds while preserving product yields. Depending on the process configuration, acetylene hydrogenation can be placed in either a front-end (with deethanizer or depropanizer first in the separation sequence) or a back-end (with demethanizer first in the separation sequence) location.
The ethylene/ethane mixture is sent to an ethylene/ethane splitter, also known as a super fractionator, where pure ethylene is separated and collected (Figure 6). Any remaining ethane is recycled back to the furnace for further cracking. Final purification steps ensure ethylene meets stringent quality specifications for downstream derivative production.

▲Figure 6. The recovery section of the ethylene production process separates and purifies the products from the cracked gas stream. Recovery involves cryogenic separation of the gas in a series of distillation columns — ultimately producing high-purity ethylene. Shown here is the recovery section of the Borouge 2 Ethylene Plant in the United Arab Emirates. Photo courtesy of Linde Engineering.
Similarly, the propylene/propane mixture enters a propylene/propane splitter, where high-purity propylene is recovered. Any unconverted propane is recycled back to the furnace for reprocessing.
The process also recovers valuable byproducts, such as:
- Hydrogen: used in other plant processes or as a fuel source.
- Mixed C4s (including butadiene) and pyrolysis gasoline (including benzene): sold as chemical feedstocks for the production of synthetic rubber and other applications.
- Methane and other light gases: utilized as fuel for the cracking furnaces, ensuring minimal waste and maximum energy efficiency.
The ethylene manufacturing process is a highly energy-intensive process, and the specific energy consumption for the central processing steps can vary from 6,000 Btu/lb to 11,000 Btu/lb of ethylene (3,300–6,100 kcal/kg of ethylene), depending on the feedstock. The ethylene manufacturing process is complex and highly capital-intensive. The total installed cost of a world-scale ethylene plant can range from $3 billion to $4 billion, depending on the feedstock. This highly optimized process ensures the efficient production of ethylene and other valuable petrochemicals, supporting various industrial and consumer applications.
Safety considerations
The steam cracking process involves high temperatures, high pressures, and hazardous chemicals and hydrocarbons. These pose risks related to pressure hazards, flammability and explosivity, toxic exposure, material degradation and corrosion, and environmental hazards (6). The hydrocarbon industry is aware of these risks and their associated mitigation techniques. To ensure safe operation, risks in ethylene production must be systematically identified, assessed, and managed through a combination of engineering controls, process safety measures, and best operational practices. These also include comprehensive safety training and emergency preparedness.
Fired steam cracking furnaces face combustion safety challenges because of high heat loads, elevated temperatures, flammable gases, and intricate burner configurations. These require a thorough understanding of reliable ignition sources, flame monitoring, and flame stability, particularly during high-risk operations such as start-up and transient operations (6, 7).
Ethylene plants handle light hydrocarbons, exposing them to auto-refrigeration, which comes with related risks of brittle fracture. Therefore, this necessitates careful material selection, adequate operational controls, and robust emergency response measures. Preventing sudden depressurization or loss of heat source and ensuring cryogenic compatibility are critical to avoiding catastrophic failures (8).
Both the acetylene and MAPD hydrogenation reactors are highly sensitive to temperature, pressure, and reaction kinetics. Uncontrolled conditions can lead to thermal runaway, polymerization, decomposition, and potentially explosive reactions. These risks are mitigated through the implementation of engineering controls, process safety measures, and high-integrity shutdown systems.
The energy transition
As the world grapples with the realities of climate change, the petrochemical industry stands at a critical crossroads. Many chemical and petrochemical companies have set net-zero targets covering operational (Scope 1 and 2) emissions; thus, the challenge lies in reshaping operations to reduce emissions, enhance sustainability, and align with a low-carbon future (9). Ethylene manufacturing is considered hard-to-abate due to its high-temperature reaction system. Achieving net-zero carbon emissions will require a multifaceted approach, including phasing out inefficient assets, decarbonizing existing infrastructure, and building new, carbon-neutral facilities to meet growing demand. The industry’s ability to adapt and innovate will determine its role in a more sustainable future.
Reducing greenhouse gas emissions is just one aspect of the broader transition to sustainability. The petrochemical sector must also adopt efficient resource utilization, promote circularity, prioritize water conservation, and ensure responsible land use. Furthermore, a just transition must be ensured — climate action should not disproportionately impact vulnerable communities or create new economic disparities. The industry’s sustainability journey must strike a balance between environmental responsibility and economic and social considerations, ensuring resilience and long-term viability.
Meeting net-zero targets will rely on both near-term and long-term technological advancements. The industry can significantly reduce its carbon footprint by enhancing energy efficiency, adopting carbon capture and sequestration technologies, and integrating renewable energy sources. The successful implementation of these technologies will be crucial in meeting climate commitments while maintaining production efficiency.
Several proven technologies are ready for immediate deployment, helping the petrochemical industry meet intermediate decarbonization targets:
- Low-emission designs: enhancing process efficiency and yield in high-temperature operations, such as steam cracking and syngas production.
- Blue hydrogen: utilizing hydrogen produced with pre-combustion carbon capture to reduce emissions.
- Post-combustion carbon capture: capturing and storing or utilizing carbon emissions from industrial processes.
- Carbon-free electricity: transitioning to renewable energy sources to power operations.
- Renewable feedstocks: incorporating biobased and circular feedstocks into production.
- Circularity: recycling and converting waste plastics into valuable raw materials, minimizing dependence on fossil fuels.
After 2030, the chemical industry will likely use new technologies that take advantage of carbon-free electric power. One significant change may involve switching from fossil fuel-based systems to electric reactors that provide the heat needed for chemical reactions. These electric systems are more energy-efficient than traditional steam cracking. One approach is to use radiant coils, like in today’s furnaces, but heat them with electricity instead of burning fuel. Another option is shockwave reactors, which use fast-spinning blades powered by electricity to turn motion into heat.
Using electric reactors will also mean less steam is recovered. As a result, large steam-driven turbines (used for gas compression or refrigeration) will need to be replaced by powerful electric motors, capable of handling 100 MW or more.
Beyond 2035, the industry must implement transformative solutions that redefine carbon utilization and reduce emissions. These technologies might include:
- Utilizing captured CO2: converting captured carbon dioxide into valuable products, such as olefins, to support circularity.
- Chemical looping reactors: deploying innovative reactor designs to eliminate greenhouse gas emissions from industrial processes.
- Next-generation materials and processes: investigating emerging technologies that can transform the production and utilization of petrochemicals.
Meeting net-zero goals is not solely a technological challenge; it requires a robust policy framework. Governments play a crucial role in driving change through incentives, regulatory frameworks, and financial support for research and infrastructure development. Simultaneously, increased private investment in transition technologies will be crucial for scaling up low-carbon solutions and meeting the growing demand for sustainable products.
Closing thoughts
For the petrochemical industry, shifting to a low-carbon future is not merely a regulatory obligation but an opportunity to drive innovation, enhance competitiveness, and contribute to a more sustainable world. By embracing new technologies, optimizing resource efficiency, and integrating sustainability into every aspect of operations, the industry can successfully navigate the energy transition and play a vital role in global net-zero efforts.
Literature Cited
- Kapur, S., “Story of Ethylene Production: The Steam Cracking Process,” Olefins Basics, https://www.apexpetroconsultants.com/olefins-basics (May 28, 2024).
- Kapur, S., et al., “ENI Encyclopedia of Hydrocarbons,” Chapter 10.5 “Ethylene and Propylene,” Vol. II, Refining and Petrochemicals, ENI: Istituto della Enciclopedia Italiana, Rome, Italy (2006).
- Kapur, S., “Handbook of Petrochemicals Production Processes,” 1st ed., Chapter 6.1, “ABB Lummus Global SRT Cracking Technology for the Production of Ethylene,” McGraw-Hill Education, New York, NY (2005).
- Kniel, L., et al., “Ethylene – Keystone to the Petrochemical Industry,” Marcel Dekker, New York, NY (1980).
- Albright, L. F., et al., eds., “Pyrolysis: Theory and Industrial Practice,” Academic Press, Cambridge, MA (June 11, 1983).
- Van Der Eijk, J., et al., “Pragmatic Approach to Retrofitting Cracking Furnace Firing Systems for Improved Safety,” Proceedings of the Ethylene Producers’ Conferences (2013).
- Musallam, W., “Overview of Process Safety Issues in Ethylene Plants - Ethylene Furnace Safety during Transition Periods,” Proceedings of the Ethylene Producers’ Conferences (2007).
- King, R., “Auto Refrigeration-Brittle Fracture Prevention Learning Module,” Proceedings of the Ethylene Producers’ Tools and Modules, https://epc.omnibooksonline.com/change (2016).
- Kapur, S., “APEX PetroConsultants, LLC: Blogs,” https://www.apexpetroconsultants.com/blog (accessed Apr. 29, 2025).

Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
