The U.S. pharmaceutical industry faces many manufacturing challenges due to the complexity of new drug molecules and the high costs and time required to bring them to market. In particular, the small-molecule sub-sector struggles with the unpredictable flow behavior of active pharmaceutical ingredient (API) powders during manufacturing. Small molecules have a molecular weight under 900 Da and simple, stable structures; the flowability of small-molecule APIs in powder form cannot be easily described by fundamental physics-based models. That leads to a reliance on empirical methods that require data calibration using large quantities of scarce and costly APIs during process development.
For oral solid-dosage pharmaceutical manufacturing, powder flowability is a critical factor that affects processes such as mixing, feeding, die filling, and tableting. Flowability is impacted by factors such as particle size distribution, shape, density, surface chemistry, and roughness. The current practice for determining flow characteristics is to rely on empiricism, which comes with high costs. Predictive models for powder flowability could help address this significant pain point.
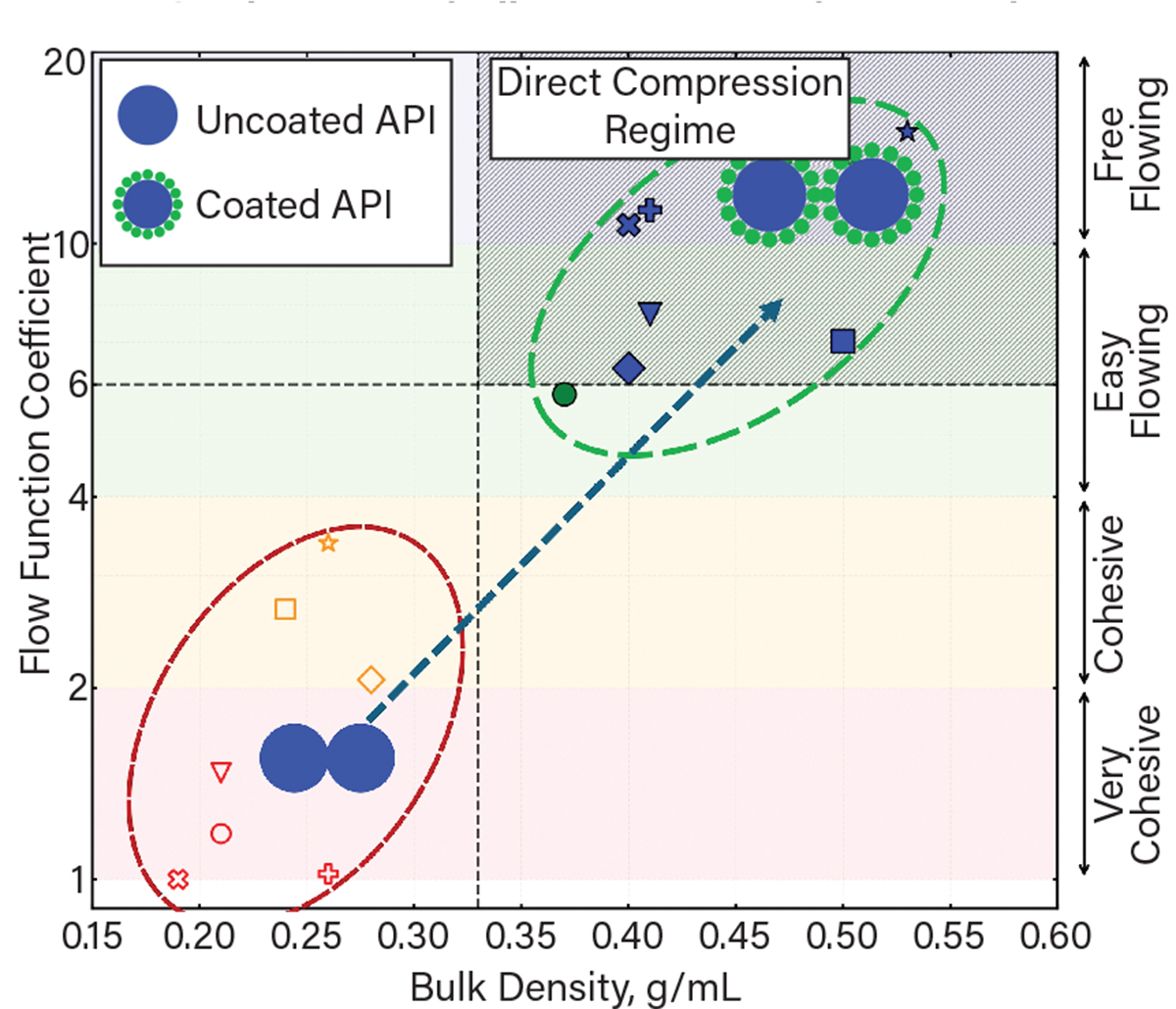
▲ Dry coating produces well-coated particles, enhancing flowability and bulk density compared to uncoated particles. Seven fine (5–20 μm), cohesive, active pharmaceutical ingredient (API) powders — represented above as circles, squares, triangles, diamonds, crosses, exes, and stars — become easy- to free-flowing after dry coating, representing remarkable enhancements that could enable direct-compression tableting, mitigating the need for a granulation process.
The New Jersey Institute of Technology (NJIT) team at the Center for Integrated Materials Science and Engineering of Pharmaceutical Products (CIMSEPP), an Industry-University Cooperative Research Center funded by the U.S. National Science Foundation, has developed mechanistic particle contact models for predicting particle cohesion and flowability. Based on van der Waals (vdW) interactions, the models link particle-scale and bulk-scale properties, and they require measuring only particle size, roughness, and surface energy, minimizing the need for large quantities of API materials for testing.
The models explain why layering nanosized flow additives or glidant particles onto fine APIs via a process called dry coating enhances their flowability. Dry coating involves using specialized high-intensity mixing devices to evenly disperse and attach highly agglomerated nano additives onto the surface of the fine API powders. The models show that the vdW forces between two naturally rough particles are highly nonlinear functions of the particle size and surface roughness, making it difficult to predict flowability. In contrast, the superimposed nanoscale roughness (~20 nm) due to dry coating linearizes the vdW forces, lowering cohesion by one to two orders of magnitude.
The team validated the flowability enhancement models using fine drug powders (5–30 μm) at lab scale, and demonstrated tunable, prediction-based flowability enhancements. The experiments showed that dry-coating API in a blend of uncoated powders results in significant property enhancements due to the transfer of silica glidant onto uncoated powders during blending. Such synergistic enhancements also increase control of the API’s concentration, which simplifies the manufacturing and tableting process. The studies confirmed that dry coating would allow for the production of smaller-sized tablets for easier oral ingestion and enhanced patient compliance.
NJIT’s dry coating research, which led to patent US 12,076,440 B2, was supported by funding from CIMSEPP’s industry members. CIMSEPP Director Rajesh Davé, his doctoral student Siddharth Tripathi, and Dr. Sebastian Escotet-Espinoza of Merck & Co. developed a collaborative pilot-scale research project supported by the NSF INTERN program. The ensuing six-month investigation demonstrated at a 10-fold scale-up that dry coating of fine APIs like acetaminophen yielded significant flowability enhancements. Remarkably, there were also substantial enhancements in downstream processability in acetaminophen blend feedability, tablet weight variability, and tablet strength. Escotet-Espinoza stated, “Siddharth’s work scaling lab dry coating to pilot-scale manufacturing gave us insights on how we could implement the technology to reduce unit operations, improve process robustness, and enhance product quality in an industrial setting.”
This research is funded by the NSF Industry-University Cooperative Research Centers program and the NSF INTERN program.
This article was prepared by the U.S. National Science Foundation in partnership with CEP.

Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
