Since their discovery more than a century ago, antibodies have become increasingly important in science and medicine. With this increased importance comes the need for rapid, convenient determination of antibody concentrations. Whether for diagnostic devices to accompany existing antibody drug therapies, use within pharmaceutical production facilities to provide feedback during manufacturing, or for improved devices for point-of-care measurement of antibody responses to disease antigens, strategies for determining specific antibody levels in minutes without interference from other proteins or antibodies are vital. Existing lateral flow assays to detect antibodies are not quantitative, and other immunoassays take hours to complete.
The National Science Foundation (NSF) Center for Bioanalytical Metrology (CBM) is working to address the challenge of point-of-care antibody analysis. This collaborative center consists of three university sites and 12 corporate members that investigate new approaches for addressing key measurement science needs of industry.
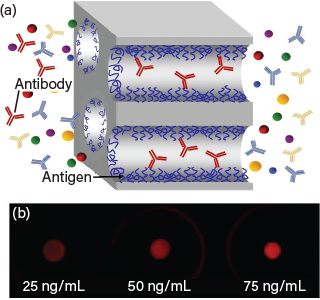
▲ (a) Functionalized membranes allow immobilization of antigens to selectively capture the antibody of interest. (b) After capture of Avastin and a fluorescently labeled secondary antibody, fluorescence imaging enables determination of Avastin concentrations.
CBM has facilitated the collaboration of chemical engineers from the Univ. of Notre Dame who study functionalized membranes with biomedical engineers from Purdue Univ. who specialize in microfluidic devices. Together, they employ innovative technologies that allow functionalization of membranes to bind specific antibodies. Subsequent capture of a fluorescently labeled secondary antibody allows the researchers to measure the concentration of the primary antibody in the initial solution. The membrane-based system has a low rate of nonspecific binding, which often plagues antibody-based assays and creates false positives and erroneous results.
Generation of fluorescence throughout the volume of the porous, functionalized membrane may increase the fluorescent signal generated by two orders of magnitude relative to a flat surface in a conventional analysis well. Membrane-based formats show promise for effective testing in as little as 5–10 min.
The Purdue Univ. team of biomedical engineers, led by Jacqueline Linnes, is developing microfluidic devices that incorporate the new functionalized membranes. They incorporated a filter in a microfluidic device upstream of an antibody-capture membrane to remove red blood cells, as colored blood cells may interfere with or decrease a fluorescent signal. The next step is simplifying the required assay process to produce streamlined tests that can be used at the point-of-care without extensive laboratory instrumentation.
The antibody assay system is surprisingly effective, easily measuring therapeutic levels of the monoclonal antibody drugs Avastin and Herceptin in the presence of serum and other potential interfering substances. The CBM team is determining whether a smartphone-based handheld fluorescent reader previously developed by the Linnes group and colleagues would be practical for measuring the membrane system’s fluorescence.
“This point-of-use antibody assay platform is a wonderful example of the NSF Industry-University Cooperative Research Centers program, which enables collaboration between the private sector and academia,” states Paul Bohn, Site Director, NSF CBM Center. Team members are currently investigating opportunities for commercializing the technology.
At the start of the COVID-19 pandemic, CBM obtained funding from NSF to extend the project to address rapid point-of-care detection of anti-SARS-CoV-2 antibodies. Typically, disease-specific antibodies develop within a week or two of infection, and they can persist for various lengths of time. While not as useful as PCR-based tests for quantifying the presence of the pathogen, point-of-care tests for the presence of anti-SARS-CoV-2 antibodies can determine if patients were previously infected, if they have developed a suitable antibody response to prior infection or vaccination, or if they are experiencing fading immunity. Similarly, assays for monoclonal antibodies that treat SARS-CoV-2 may also prove useful. The team has developed the components of a diagnostic test for a monoclonal antibody that recognizes the spike protein of the SARS-CoV-2 virus.
This research is funded though the NSF’s Industry-University Cooperative Research Centers (IUCRC) Program.
This article was prepared by the National Science Foundation in partnership with CEP.

Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
