Enzymes are key to unlocking modern-day biotechnology, whether as an additive in detergent, a step in a biosynthetic pathway, or a replacement for chemical catalysis. Traditionally, producing enzymes requires cells and cellular fermentation. Although cellular fermentation is effective for producing large quantities of certain enzymes, it may not be the best solution for discovering new enzymes. Researchers have searched for a simpler method — in essence, a prototyping system — that could accelerate the discovery process without requiring fermentation.
In 2012, Richard Murray, a bioengineer at California Institute of Technology (Caltech), and graduate student Zachary Sun demonstrated a method of prototyping biological circuits without fermentation. “A key element of the design process in areas such as aeronautical, mechanical, and electrical engineering is the ability to perform rapid prototyping of early design concepts, and this was missing for biological engineering,” said Murray. Murray and Sun created a “biomolecular breadboard” based on a cell-free system of crude lysates derived from E. coli, which are capable of transcription and translation. Consequently, they are able to speed engineering cycles multifold for building complex genetic circuits such as feedforward loops and oscillators.
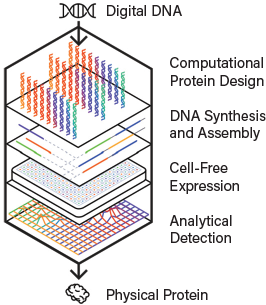
▲ Tierra Biosciences uses cell-free systems to conduct experiments that yield physical enzymes for testing. The platform can be used to produce enzymes and other proteins at high throughputs.
“Seeing the success of our cell-free prototyping concept, I thought, ‘Could we apply this to the discovery and engineering of enzymes?’” says Sun. He theorized that eliminating the need for cloning, transformation, and growth of cells could yield multifold improvements in scale, time, and costs for producing enzymes and identifying candidates to move forward. Sun cofounded Tierra Biosciences in part to perfect the science of cell-free systems; today, he serves as the company’s CEO.
Although the cell-free prototyping technology harnessed by Tierra stems from the earlier Caltech system, it needed to be adapted to fit the enzymes market, where producing enough material for downstream assays is key. With National Science Foundation (NSF) support, Sun and research collaborator Philip Romero, a biochemist at the Univ. of Wisconsin-Madison, evolved the technology into Tierra’s novel pipeline.
The pipeline consists of a completely ex vivo process of computational protein design, DNA synthesis, DNA assembly, cell-free expression, and analytical detection methods. The process yields not only data on enzyme behavior but also physical enzymes for downstream work. To demonstrate the platform, Sun and Romero engineered and produced cytochrome P450s, which are a historically difficult class of enzymes to produce in cells.
The platform leverages two particular advantages of cell-free synthetic biology: Inputs can be linear DNA pieces, and therefore do not need to be cloned into plasmids; and because the cell-free platform is not restricted by a cell wall, protein production can be seen in real time in a way not feasible with cells.
Multiple companies currently partner with Tierra to leverage high-throughput protein expression in their discovery pipeline. “Tierra’s platform, and especially their cytochrome P450 engineering capabilities, have been critical in our efforts to develop a novel antimalarial drug production process. This enzymatic biocatalysis process, wherein the main bottleneck is a cytochrome P450 enzyme, is toxic to cells and hard to debug without Tierra’s platform,” says Deepak Dugar, CEO at Visolis and one of Tierra’s early customers.
Tierra has taken advantage of the rapid, parallel growth of complementary technologies in the synthetic biology ecosystem. Only in the past 3–5 years have technologies like on-demand synthetic DNA (Twist Biosciences), low-cost, Python-enabled liquid handling (OpenTrons), and flexible, acoustic liquid handling (Labcyte) become widespread. These technologies now serve as a critical enabling part of the Tierra pipeline.
“While companies before needed specialized R&D teams to build bioproducts, startups are now able to make new bioproducts by leveraging existing expertise,” says Sun. “As our ecosystem continues to evolve, our bet is that cell-free synthetic biology will be key to any company’s discovery process.”
This technology was funded through the NSF Small Business Technology Transfer Research (STTR) Program.
This article was prepared by the National Science Foundation in partnership with CEP.

Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
