Biocatalysis, the use of enzymes to perform synthetic chemistry, has grown in significance. Today, enzymes are becoming part of the essential toolbox for the industrial synthesis of high-purity chemicals.
Merck & Co. and Codexis pioneered industrial biocatalysis for pharmaceuticals with a biosynthetic route to produce sitagliptin, the active ingredient in one of Merck’s anti-diabetes drugs, eliminating transition-metal-catalyzed, high-pressure hydrogenation and chiral purification steps. The new process had 56% higher productivity and 13% higher yield, and generated 19% less waste than the original process. In the past 10 years, the industry has similarly engineered more than 40 new enzymes for key synthesis steps to lower the cost and environmental impact of drug production.
However, enzymes cannot always compete economically with traditional chemistry and inorganic catalysts. For commercial-scale processes, enzymes are often immobilized on solid supports so that they can be reused. But enzyme immobilization methods can have significant drawbacks: They are not universal for all enzymes, have poor immobilization yield, drastically reduce enzyme activity, and can involve the use of expensive support materials.
If done effectively, though, immobilization can be advantageous. Biocatalysts are often more stable when they are immobilized. For example, they can tolerate organic solvents and higher temperatures, and can be used and reused efficiently over multiple synthesis cycles.
“Although many examples of enzyme immobilization exist, a general methodology has yet to be developed,” says Matthew D. Truppo, head of chemical biotechnology at Merck. “The development of a general immobilization protocol that can be applied readily and inexpensively across multiple enzyme classes is of great interest,” Truppo says.
ZYMtronix, an Ithaca, NY-based spinout from Cornell Univ., has developed a universal method and associated materials that address the shortcomings of today’s enzyme immobilization techniques. The method involves magnetic nanoparticles (5–10-nm dia.) that self-assemble into magnetic clusters to permanently entrap and stabilize the enzymes. The enzyme-cluster combinations may be magnetically coupled with a magnetic macroporous carrier (support) for ease of use.
“Our platform is universal and allows for quick customization of magnetic carriers around any wild-type or engineered enzymes, or their combinations, while ensuring the highest activities,” says Stéphane Corgié, CEO of ZYMtronix.
The company approaches enzyme immobilization in three steps. Because each enzyme is unique, the first step is a quick optimization to find the right enzyme-to-nanoparticle ratio to form the clusters. The second step involves screening the clusters to identify the immobilized enzymes with the highest activities. Once identified, enzyme-cluster candidates are assembled with the magnetic carriers and produced at the volume needed to meet a client’s needs. In the final step, ZYMtronix develops and provides the client fitted magnetic tools for their equipment to mix, capture, and recycle the enzyme-cluster-carrier assemblies.
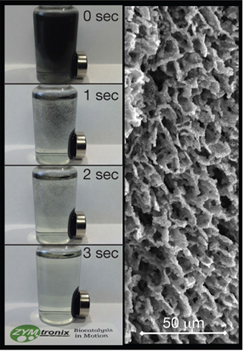
▲ A scanning electron micrograph of a magnetic particle of ZYMtronix’s new ZYM-MAG material reveals the hierarchical structure of the macroporous enzyme carrier. Even the finest ZYM-MAGs (< 200-µm size fraction, shown in the vial) can be captured in seconds by external magnetic fields (here, a magnet on the side of the vial) thanks to their high magnetic susceptibility.
ZYMtronix has successfully applied its immobilization technique to more than 40 enzyme systems, with no reductions in enzyme activity, at loadings above 20 wt% (weight of enzyme to weight of carrier). For comparison, competing immobilization technologies seldom reach enzyme loadings of 10%, and activities typically decrease by as much as 80%.
With support from the National Science Foundation, ZYMtronix has scaled up its process to grams of immobilized enzymes per day, and has demonstrated the use of the immobilized enzymes in both batch and continuous processes. To accelerate the adoption of its technology, the company recently launched rapid magnetic optimization kits. The kits come in a 96-well microplate format and are loaded with the client’s immobilized enzymes to be tested on their substrates of choice. The kits can be adapted for synthetic biology involving synthesis with multiple enzyme systems.
The company is currently in discussions with strategic partners to test the technology at larger scales.
This technology was funded through the NSF Small Business Innovation Research Program.
This article was prepared by the National Science Foundation in partnership with CEP.

Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
