Various laboratory experimental techniques are recommended for developing a safe chemical process, many of which evaluate heat and pressure generation during runaway reactions through the use of calorimetry.
Reactive chemical hazards are a special subset of chemical hazards that can arise whether the reaction is intended or not. Inadequate safeguards or unknown hazards can lead to catastrophic consequences such as explosions, fires, or harmful chemical releases.
Selecting the best experimental approach to study reactive hazards involves identifying the objective of the assessment and understanding potential equipment limitations for a given reaction. Calorimeters are the cornerstone of these experiments, as they are capable of analyzing the thermal behavior of chemical processes. This article overviews heat flow calorimeters and adiabatic calorimeters — powerful instruments that provide valuable data for safe process design.
In order to determine the best experimental approach, the objective of the assessment must first be considered. For example (Figure 1), the goal might be to prevent a reaction from occurring by determining safe operating or storage temperatures, mitigate the consequences of an upset condition with an adequate emergency relief system, or eliminate the reaction from occurring with safeguards or procedures. Or, the goal might be to determine the likelihood of a reaction occurring or understand the severity or consequence if the reaction does occur. Depending on the specific objective, a variety of metrics — such as heat of reaction, adiabatic temperature rise, instantaneous heat generation, adiabatic time to maximum rate (TMR), self-accelerating decomposition temperature (SADT), emergency relief requirement, and more — can be evaluated through experimentation to help meet these goals. Each metric may have different experimental techniques that are optimal for collecting the data required for its calculation.
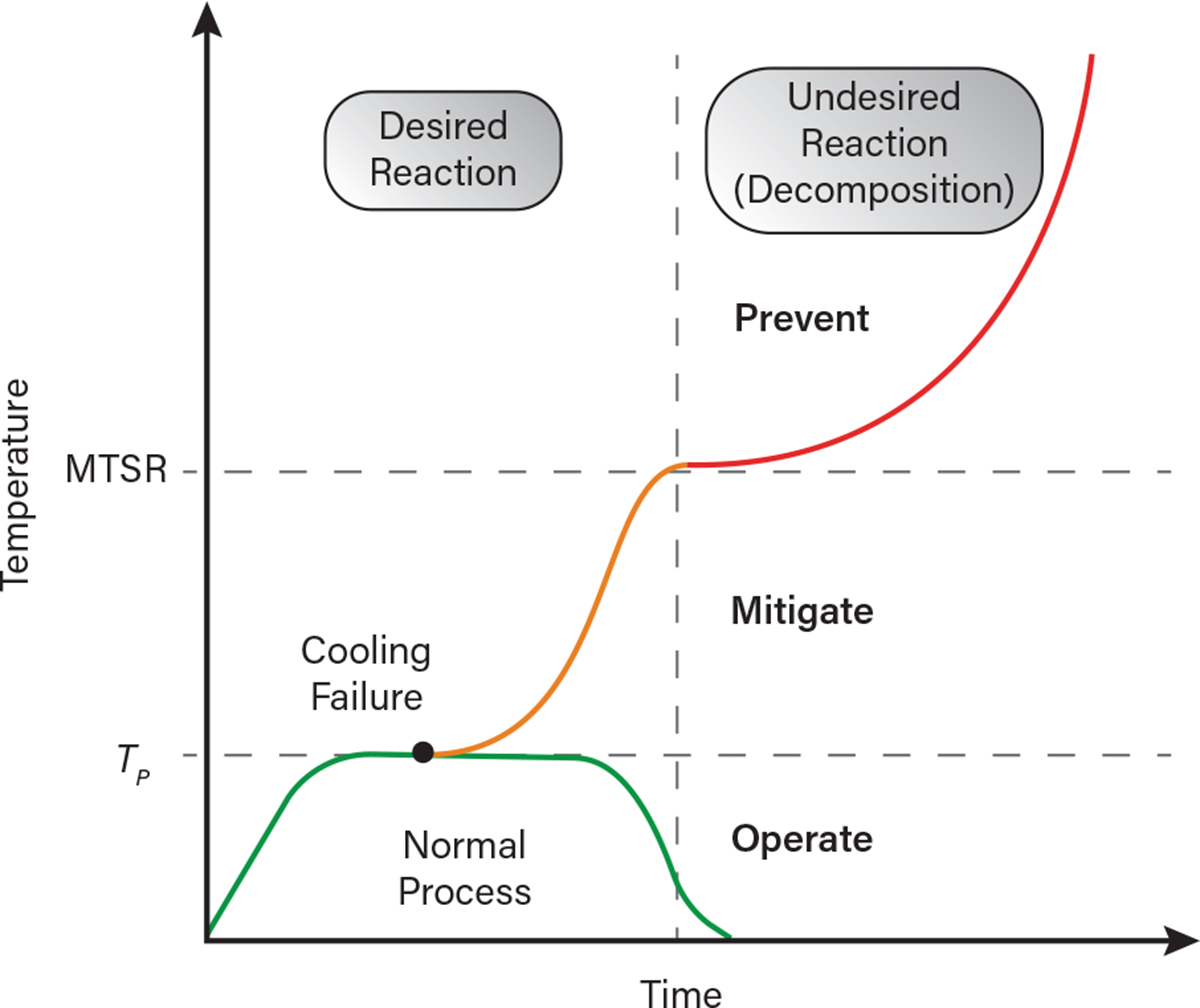
▲Figure 1. If a cooling failure occurs during normal operating conditions at the process temperature (TP), the intended reaction may result in the temperature rising to the maximum temperature of the synthesis reaction (MTSR). It is crucial to understand if a secondary reaction is lying in wait above this temperature; if so, this undesired reaction should be assessed using calorimetry data, and the resulting consequences should be mitigated or prevented depending on the hazard. Source: (1).
Then, the reaction details should be reviewed and compared to potential equipment limitations. Factors such as the temperature range, pressure range, sensitivity, material of construction and compatibility, sample size, stirring, and desired or undesired reaction procedure can all change the recommended approach. Different experimental approaches have different capabilities and limitations, and it is important to select the right tool to successfully study the reactive hazard.
The next sections provide an overview of laboratory techniques and best practices for data collection for assessing reactive hazards and developing safe chemical processes...
Would you like to access the complete CEP Article?
No problem. You just have to complete the following steps.
You have completed 0 of 2 steps.
-
Log in
You must be logged in to view this content. Log in now.
-
AIChE Membership
You must be an AIChE member to view this article. Join now.
Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
