Sections
Synthetic opioids have caused a sharp increase in deaths over the past five years, reinforcing the need for immediate solutions from the medical and chemical engineering communities.
The opioid epidemic in the U.S. is one of the largest public health emergencies of our time. Opioid overdoses have triggered a mortality crisis in the U.S., and opioid addiction is a major cause of declining life expectancy. Moreover, the opioid crisis threatens to spread to middle-income and low-income countries, where the use of opioid drugs is rapidly proliferating. This article, a follow-up to a CEP article published in November 2017 (1), describes the history of the opioid epidemic and reviews some of the challenges of providing community education and combating negative stigma. The article discusses the specific actions that chemical engineers can take together with physicians to battle the opioid crisis.
A brief history of the opioid epidemic
The opioid epidemic in the U.S. is traditionally split into three main phases driven by different sources of opioid deaths: the first being prescription opioids, followed by heroin, and most recently synthetic opioids such as fentanyl (Figure 1) (2, 3). The first phase was greatly aided by lobbying efforts to prioritize pain in the medical field, along with misleading advertising of Purdue’s controlled-release opioid, OxyContin, in 1995. OxyContin was liberally prescribed in part due to the inaccurate belief that it had reduced abuse potential. We are now aware that OxyContin, like all other opioids, has high abuse liability and potential for dependence (4). It is estimated that 8–12% of people who take opioid prescription medications for chronic pain go on to develop opioid use disorder (OUD).
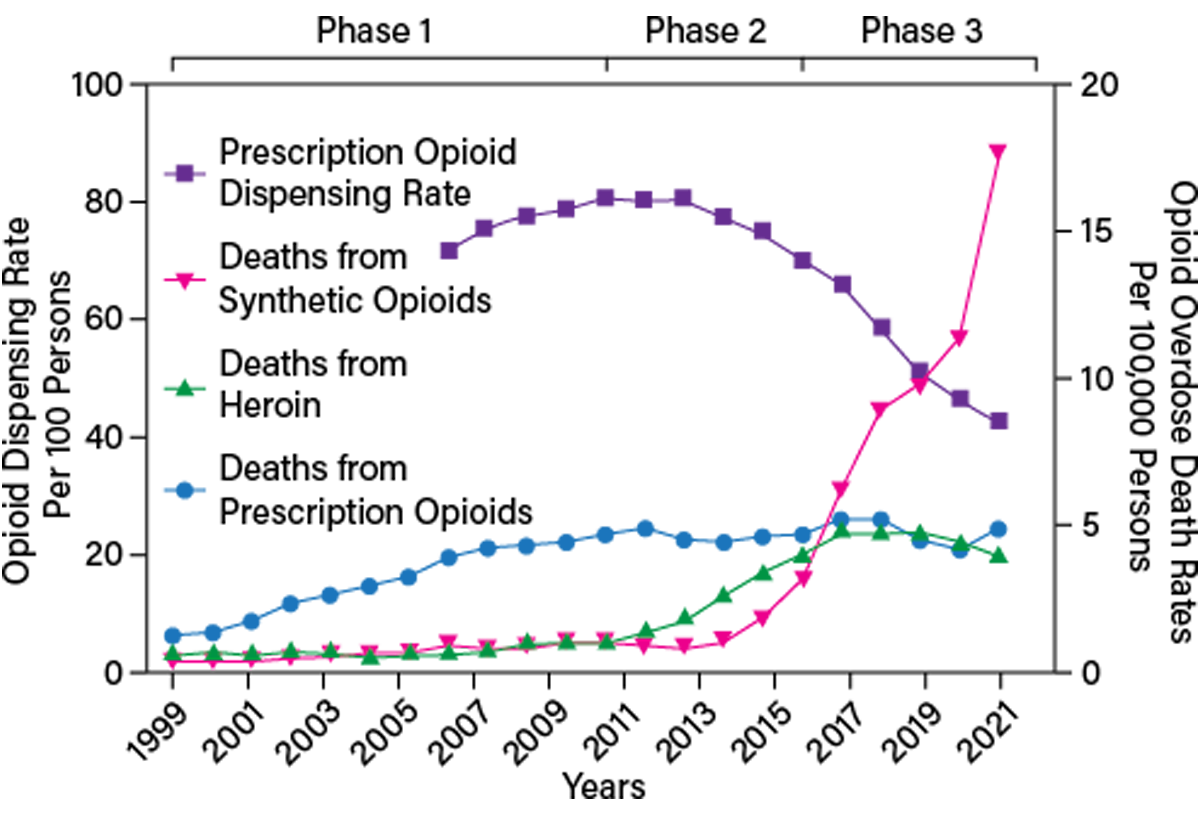
▲Figure 1. The opioid epidemic in the U.S. is traditionally split into three main phases driven by different sources of opioid deaths: prescription opioids, heroin, and synthetic opioids such as fentanyl. Source: Adapted from (2, 3).
As overdose deaths from prescription opioids steadily rose throughout the 2000s, the number of opioid prescriptions began to decline in the late 2000s. Despite efforts to curtail opioid prescriptions, by 2009, there were more than one million emergency department visits due to misuse of pharmaceutical drugs, outpacing visits from heroin and cocaine. In response, the volume of prescription opioids dispensed has been greatly reduced between 2009 and 2019, from 80 prescriptions per 100 persons down to 40 prescriptions per 100 persons in the U.S., respectively (Figure 1, purple squares) (2, 3).
The lowered rates of opioid prescriptions coincide with the second phase of the opioid epidemic: increased heroin overdose deaths (Figure 1, green triangles). Today, an estimated 80% of people who use heroin begin by first misusing a prescription opioid. Heroin costs significantly less than prescription opioids and is more accessible. In 2005, less than 10% of individuals who regularly used opioids began by using heroin; however, by 2015, there was a three-fold increase in this number (5).
One key difference between heroin and prescription opioids is that the unregulated nature of heroin translates into individuals not being certain of the potency of the heroin they consume. Another important difference between heroin and prescription opioids is the route of administration, which influences the potential addictive properties of the opioid. Most individuals that use prescription opioids administer them orally. Oral administration has a relatively lower risk for addiction than intravenous administration, which is the route many individuals administer heroin. Thus, heroin’s properties induce tolerance and addiction faster than orally delivered opioids.
In the third and current phase, heroin has been combined with or replaced by synthetic opioids. Similar to heroin, obtaining accurate dosing of these potent synthetic opioids is very challenging, if not impossible; thus, overdoses are very common. Deaths from synthetic opioids such as fentanyl have skyrocketed from 2015 to 2021, especially during the social isolation of the COVID-19 pandemic (Figure 1, pink triangles).
Importantly, it is reported that most individuals do not want to use heroin that is combined with (i.e., “cut with”) fentanyl, but fentanyl is nevertheless inundating the illicit opioid market. The rise in prevalence of fentanyl, and therefore increased overdoses from fentanyl, is largely due to the combination of fentanyl’s high potency and low cost. Fentanyl can be easily added to heroin, making heroin sales more profitable (6). Similar to the difficulty of knowing the purity or strength of heroin, the same risk is amplified for fentanyl-spiked heroin because fentanyl is 30–50-fold more potent than heroin. This means that the amount of drug required to potentially cause an overdose is up to 50-fold lower when fentanyl is added to heroin compared to heroin alone.
While the number of opioid prescriptions continues to decline, overdose deaths from prescription opioids have not. Rather, deaths from prescription opioids have plateaued (Figure 1, blue circles). Following a similar trend, despite this recent rise in synthetic opioid deaths, death rates from heroin overdoses have not decreased but have remained constant (Figure 1, green triangles). Taken together, these disconcerting trends suggest that the first two phases of the opioid epidemic have not been adequately addressed. Instead, the continued rise in overdose death tolls is overshadowed by the latest phase.
The most recent Diagnostic and Statistical Manual of Mental Disorders handbook combines substance abuse and dependence into one collective diagnosis as substance use disorder with varying levels of severity from mild to severe, depending on the number of criteria met. If the drug that contributes to the diagnosis of a substance use disorder is an opioid, the diagnosis is deemed OUD.
Each individual’s experience with OUD will be different; however, there are some common aspects to developing an OUD. Initially, individuals are exposed to or experiment with opioids, which produces substantial euphoria. The most common initial opioid is a prescription opioid such as oxycodone or hydrocodone (7). Opioid consumption often escalates over time, potentially leading to tolerance, dependence, and/or the appearance of withdrawal symptoms. Individuals with OUD often experience physical withdrawal symptoms as well as dysphoria that can last for days following cessation of opioid use. At this point, individuals are often forced to alleviate these symptoms by any means necessary, and consuming opioids leads to at least partial remedy of these negative aspects. Following substantial tolerance to opioids, administering the drug merely removes the withdrawal symptoms and no longer produces the euphoria that characterizes the initial drug use.
A key feature of OUD, similar to other substance use disorders, is the chronic relapsing nature. In 2020, it was estimated that almost 2.5 million individuals suffered from OUD. Of all drug overdose deaths, a staggering 75% of them involved an opioid (7), highlighting how incessant opioids are in driving overdose deaths.
The causes of OUD are not fully understood, but various genetic and environmental factors can dictate an individual’s likelihood of developing this disorder. For example, multiple studies have found significant associations between specific genetic mutations and OUD (8–10). Successful treatment of OUD is perceived as difficult. Treatment with medically supervised withdrawals alone is common but not sufficient because aspects beyond the physical withdrawal also need to be addressed, such as prolonged drug craving. Evidence-based research indicates OUD is a highly treatable condition when individuals with OUD receive medication for OUD (MOUD) (11).
Millions of people in the U.S. with OUD are not obtaining effective treatment. With ten people dying from opioid overdoses every hour in 2021, and deaths continuing to rise, the current approach to providing care for people with OUD must not be working. We aim to identify areas ripe for reassessment and innovation to substantially decrease the rates of overdose deaths in this country.
Lack of community education
Compared to the magnitude of the opioid epidemic, not enough time is dedicated to training community members and families on how to approach people with OUD. Individuals with OUD often suffer from undiagnosed anxiety or depression and/or experienced adverse childhood events (12). Many individuals use opioids to cope with their undiagnosed conditions. Rather than passively let individuals with OUD self-medicate, psychiatric evaluations in a supportive environment should address individuals’ needs and be used as a basis for effective treatment plans.
Current community outreach education such as narcan overdose reversal training for healthcare, law enforcement agencies, and the general public does not adequately highlight the efficacy of MOUD for individuals with OUD. MOUDs such as methadone, buprenorphine, and naltrexone are highly effective once-daily oral medications to treat OUD (13). The concept of harm reduction derived from MOUDs should be underscored within the community education curriculum. For example, buprenorphine is a partial opioid agonist with effects that can last 1–3 days, which is considered long-acting. It prevents individuals with OUD from developing severe withdrawal symptoms when they stop using short-acting opioids like heroin, with effects lasting only up to six hours. While this medication is still an opioid, it avoids the inherent risks of injection-based opioid delivery, including Hepatitis C, HIV, or septic shock from a skin abscess. In addition, the risk of a fatal overdose significantly decreases when individuals receive standardized doses of medication from federally regulated pharmaceutical manufacturers. Thus, MOUD for OUD reduces the burden on hospital emergency rooms, lowers the cost of treating secondary infections, and prevents overdose mortality.
MOUD access should be expanded, as others have suggested (14). When ER physicians initiate treatments with buprenorphine, individuals with OUD are twice as likely to enter treatment programs 30 days later. Unfortunately, legislative and stigma-based barriers to access prevent people with OUD from obtaining this life-saving medication.
The U.S. Drug Enforcement Agency (DEA) regulates buprenorphine medications and requires clinicians to apply for an “X waiver” to write outpatient prescriptions for buprenorphine. While methadone requires frequent visits to clinics and onerous demands on individuals, prescribed buprenorphine can be accessed through a local pharmacy and taken at home. Thus, an X-waivered physician can deliver effective treatment to individuals with OUD in a respectful, dignified, and unstigmatized manner. However, over half of Americans living in rural settings lack access to an X-waivered provider. Even if a provider is X-waivered, the DEA limits the number of buprenorphine prescriptions that a clinician can provide at one time. Recent legislative action has removed the mandatory training required for the X-waiver and increased telehealth access, yet retained the limits on the number of prescriptions and the requirement to register with the DEA for an X-waver identifier number. It is unfortunate that the number of opioids prescribed by a physician is virtually unlimited while the number of prescriptions to treat OUD is restricted.
Negative stigma
In addition to legislative and federal restrictions for MOUD access, there is a more insidious barrier to treatment for individuals with OUD: negative stigma. Many people hold a negative stigma toward people who develop OUD. When surveyed, three out of four primary care physicians reported they are unwilling to work closely with individuals with OUD (15). The same survey found that two out of three primary care physicians believed that individuals with OUD are more dangerous than the general population. This negative stigma experienced by individuals with OUD is pervasive across society and compounds other racial and economic injustices that individuals may face when seeking treatment. While community education cannot eliminate the negative stigma associated with OUD, it can train community members to combat OUD stigma and increase access to effective treatments (16).
The good news is that the treatment for stigma exists. Studies demonstrate that stigma is significantly reduced through training, outreach, and continuing education (16, 17). During OUD de-stigmatization training events, it is important to educate participants in a non-judgmental, evidence-based manner to help remove the years of negative connotations surrounding people with OUD. Changing community members’ views of OUD is paramount to increasing treatment access and decreasing overdose mortality from the opioid epidemic.
Engineering education
The Grand Challenges for Engineering, set forth by the National Academy of Engineering (NAE) in 2008, defined 14 priorities for engineers to address in the 21st century. In 2015, over 120 U.S. engineering schools signed a letter of commitment to President Obama to educate a new generation of engineers expressly equipped to address the Grand Challenges for Engineering. The Grand Challenges for Engineering have thus had a dramatic impact on engineering education.
Among the list of top 14 priorities is to “engineer better medicines.” Less-addictive opioid pain medicines, along with more accessible MOUDs (Figure 2) (18), certainly fall within the realm of better medicines, but these medicines have not been emphasized within this grand challenge. Indeed, the 2022 NAE report on “New Directions for Chemical Engineering” does not contain a single mention of opioids.
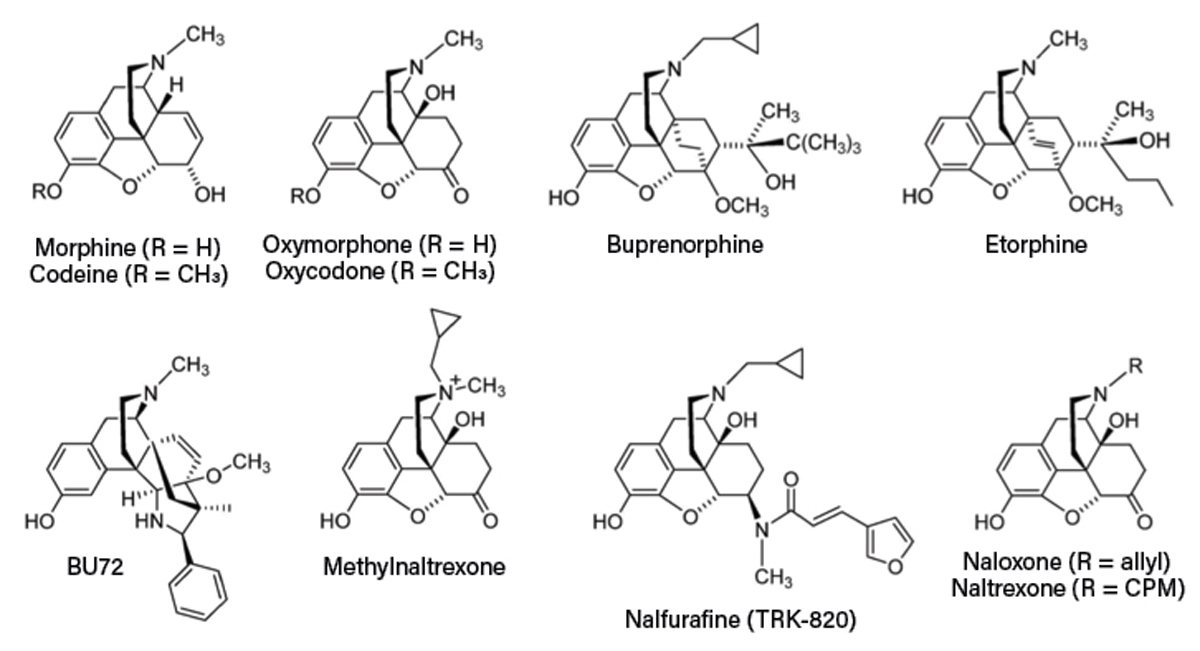
▲Figure 2. These are the chemical structures of common opioids. Source: Adapted from Ref. 18.
Chemical engineers play an important role in designing and manufacturing solutions to the opioid crisis, yet most engineering design and engineering ethics courses barely address the opioid epidemic. Chemical engineering process and product design courses could incorporate case studies of the manufacturing of methadone, buprenorphine, and naloxone, and challenge students to lower the cost and increase the availability of MOUDs. Drug delivery courses could include projects on the controlled release of opioids to minimize addiction potential.
Biochemical engineering courses should review biomanufacturing of opioids, such as the pioneering work of Christina Smolke at Stanford Univ. (19) and John Dueber at UC Berkeley (20) to manufacture natural and semi-synthetic opioids in yeast. These innovations in synthetic biology provide numerous avenues to develop less-addictive opioids. Such case studies would not only enhance students’ understanding of chemical engineering principles but also empower students to address arguably the most pressing healthcare need in the U.S. and an emerging healthcare need worldwide.
Traditional chemical engineering curricula provide few opportunities for chemical engineering students to interact with medical students and physicians. While biomedical engineering degree programs emphasize immersive design projects in which students directly interface with medical students and physicians, chemical engineering programs overlook the many opportunities for chemical engineers to contribute to medicine. Process and product design courses for chemical engineers should encourage and enable chemical engineering students to work on interdisciplinary teams with medical students; these teams would set technical specifications according to an individual’s needs and design optimal formulations, delivery routes, and manufacturing processes for less-addictive opioids and more accessible MOUDs.
Ultimately, engineering ethics courses across all branches of engineering must address the ongoing opioid crisis and the roles that engineers can play in stopping the crisis. Traditional engineering ethics courses focus on the technical feasibility of designs, rather than the impact of manufactured goods on public health. The opioid epidemic affects both health and economic welfare and thus chemical engineers have a duty to develop processes and products that alleviate the burden of opioid addiction.
Our call for action
The negative stigma blocking individuals with OUD from accessing effective MOUDs needs to be addressed to combat the opioid epidemic. In addition, we need to foster novel collaborations between physicians, researchers, and chemical engineers to address the opioid epidemic. New non-addictive medications for pain management are required to reduce the medical community’s reliance on opioids. This requires investments in basic science, chronic pain models, and biomarkers of pain (4).
When a person overdoses on opioids, they require urgent opioid-overdose reversal with intranasal naloxone to prevent death from respiratory depression. While naloxone prevents death from opioid overdose, it also induces severe withdrawal symptoms (21). In addition, the half-life of naloxone is approximately 60 minutes, which is shorter than the half-life of many synthetic opioids currently available on the street to people with OUD. Thus, naloxone-administered individuals require vigilant monitoring over the following 12 hours to ensure a subsequent overdose does not occur (22). Chemical engineers and physicians should collaborate to improve the kinetics and properties of naloxone to reduce symptoms of respiratory depression without inducing severe withdrawal, while also increasing its duration of action.
For individuals who are ready to obtain treatment for OUD, MOUDs have decades of evidence-based research supporting their efficacy when prescribed at the correct dose. More physicians should reexamine their preconceptions and beliefs surrounding individuals with OUD. Individuals with OUD are human beings stuck in a destructive loop of dangerous decisions because they lack better alternatives. It is the role of physicians to provide treatments, support, and respect for all types of individuals.
In response to the COVID-19 pandemic, federal regulations deemed telemedicine visits sufficient to prescribe buprenorphine to individuals with OUD (23). Telemedicine greatly expanded access to MOUD while also removing the barriers of stigmatization to accessing care. This innovative strategy found that 80% of individuals with OUD prescribed buprenorphine were retained in the telemedicine treatment program at the one-year follow-up (24). Telemedicine should be expanded within communities to reach people with OUD who are ready to start treatment. Needle exchange programs and safe injection sites foster opportunities for trained professionals to initiate conversations about the effective MOUD treatments available for people with OUD. More efforts are required to remove the negative stigma associated with needle exchange and safe injection sites to expand access to telemedicine and treatments for OUD.
In addition, chemical engineers and physicians should collaborate to further improve the properties of MOUDs like buprenorphine. Current strides in MOUD delivery have brought forth an implantable buprenorphine product that was approved by the U.S. Food and Drug Administration (FDA) in 2016. The delivery of MOUDs could be optimized after obtaining feedback from individuals in OUD treatment programs and physicians. As a result, the risks and side effects of MOUD could be further reduced through chemical design. Chemical engineers could improve MOUD options to prevent opioid relapses and help turn the tide of the opioid epidemic.
Effectively combating the opioid epidemic requires education, awareness, innovations, new collaborations, novel approaches, and compassion. We believe it is the duty of the medical and chemical engineering communities to end the opioid epidemic.
Literature Cited
- Bhatia, S., and D. L. Turock, “Chemical Engineers Respond to the Addiction Epidemic,” Chemical Engineering Progress, 113 (11), pp. 29–33 (Nov. 2017).
- U.S. Centers for Disease Control and Prevention, “Overdose Death Rates Involving Opioids, by Type, United States, 1999–2020,” CDC, https://www.cdc.gov/drugoverdose/data/OD-death-data.html (2022).
- U.S. Centers for Disease Control and Prevention, “U.S. Opioid Dispensing Rate Maps,” CDC, https://www.cdc.gov/drugoverdose/rxrate-maps/index.html (2022).
- Coussens, N. P., et al., “The Opioid Crisis and the Future of Addiction and Pain Therapeutics,” Journal of Pharmacology and Experimental Therapeutics, 371 (2), pp. 396–408 (2019).
- Cicero, T. J., and M. S. Ellis, “The Prescription Opioid Epidemic: A Review of Qualitative Studies on the Progression from Initial Use to Abuse,” Dialogues in Clinical Neuroscience, 19 (3), pp. 259–269 (2017).
- Ciccarone, D., et al., “Heroin Uncertainties: Exploring Users’ Perceptions of Fentanyl-Adulterated and -Substituted ‘Heroin,’” International Journal of Drug Policy, 46, pp. 146–155 (2017).
- Cicero, T. J., et al., “Increased Use of Heroin as an Initiating Opioid of Abuse: Further Considerations and Policy Implications,” Addictive Behaviors, 87, pp. 267–271 (2018).
- Gelernter, J., et al., “Genome-Wide Association Study of Opioid Dependence: Multiple Associations Mapped to Calcium and Potassium Pathways,” Biological Psychiatry, 76 (1), pp. 66–74 (2014).
- Nelson, E. C., et al., “Evidence of CNIH3 Involvement in Opioid Dependence,” Molecular Psychiatry, 21 (5), pp. 608–614 (2016).
- Hancock, D. B., et al., “Cis-Expression Quantitative Trait Loci Mapping Reveals Replicable Associations with Heroin Addiction in OPRM1,” Biological Psychiatry, 78 (7), pp. 474–484 (2015).
- U.S. Centers for Disease Control and Prevention, “Recovery is Possible: Treatment for Opioid Addiction,” CDC, https://www.cdc.gov/drugoverdose/featured-topics/treatment-recovery.html (2021).
- Derefinko, K. J., et al., “Adverse Childhood Experiences Predict Opioid Relapse During Treatment Among Rural Adults,” Addictive Behaviors, 96, pp. 171–174 (2019).
- U.S. Substance Abuse and Mental Health Services Administration, “Medication-Assisted Treatment (MAT),” SAMHSA, https://www.samhsa.gov/medication-assisted-treatment (2022).
- Deyo-Svendsen, M., et al., “Medication-Assisted Treatment for Opioid Use Disorder in a Rural Family Medicine Practice,” Journal of Primary Care & Community Health, doi: 10.1177/2150132720931720 (2020).
- Kennedy-Hendricks, A., et al., “Primary Care Physicians’ Perspectives on the Prescription Opioid Epidemic,” Drug and Alcohol Dependence, 165, pp. 61–70 (2016).
- Brown, S. A., “Stigma Towards Marijuana Users and Heroin Users,” Journal of Psychoactive Drugs, 47 (3), pp. 213–220 (2015).
- Bascou, N. A., et al., “Reducing the Stigma Surrounding Opioid Use Disorder: Evaluating an Opioid Overdose Prevention Training Program Applied to a Diverse Population,” Harm Reduction Journal, 19, #5 (2022).
- Spetea, M., and H. Schmidhammer, “Recent Chemical and Pharmacological Developments on 14-Oxygenated-N-methylmorphinan-6-ones,” Molecules, 26 (18), 5677 (2021).
- Galanie, S., et al., “Complete Biosynthesis of Opioids in Yeast,” Science, 349 (6252), pp. 1095–1100 (2015).
- Pyne, M. E., et al., “A Yeast Platform for High-Level Synthesis of Tetrahydroisoquinoline Alkaloids,” Nature Communications, 11, #3337 (2020).
- Wermeling, D. P., “Review of Naloxone Safety for Opioid Overdose: Practical Considerations for New Technology and Expanded Public Access,” Therapeutic Advances in Drug Safety, 6 (1), pp. 20–31 (2015).
- Lynn, R. R., and J. L. Galinkin, “Naloxone Dosage for Opioid Reversal: Current Evidence and Clinical Implications,” Therapeutic Advances in Drug Safety, 9 (1), pp. 63–88 (2018).
- Wang, L., et al., “Telemedicine Increases Access to Buprenorphine Initiation During the COVID-19 Pandemic,” Journal of Substance Abuse Treatment, 24, 108272 (2021).
- Harris, R., et al., “Utilizing Telemedicine During COVID-19 Pandemic for a Low-Threshold, Street-Based Buprenorphine Program,” Drug and Alcohol Dependence, 230, 109187 (2022).

Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
