(408e) CO2 Recovery from Ultra-Dilute Streams: Bridging a Novel Temperature Vacuum Swing Adsorption Model with Materials Screening
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Separations Division
CO2 Capture By Adsorption II
Thursday, November 19, 2020 - 8:30am to 8:45am
Studies attested that adsorption processes are best suited for the efficient removal of trace gas impurities aiming to achieve extremely high purities (Sircar et al., 1996). The two main reasons for this are: (a) the availability of a large spectrum of microporous adsorbents with varying pore structures and surface properties, and (b) the possibility of designing many different process schemes by tailoring generic adsorption separation methods. Materials like metal-organic frameworks (MOFs), whose chemical composition and pore shape can be optimally tuned for particular applications are good candidates, but we are currently lacking a meaningful systematic way, from a process viewpoint, to rank them for particular applications.
In our previous work, we developed a molecular simulation tool which allows us to screen thousands of materials for adsorption using their physicochemical and adsorptive properties (Boyd et al., 2019). In this work, we extend that study by coupling the molecular simulation tool with process modelling to rank materials for dilute gas separations and for a given set of process metrics, including the overall energy efficiency and process productivity. A novel 5-step temperature-vacuum swing adsorption (TVSA) process was developed, and its performance was evaluated by using an equilibrium-based shortcut approach similar to that described by Joss et al., 2015. Our TVSA process model is then coupled with molecular simulations, which allows the evaluation of both, physicochemical and adsorptive properties of thousands of microporous structures, including ~300,000 MOFs (Boyd et al., 2019).
We applied the model to a particular ultra-dilute binary mixture of N2 and CO2 (CO2 concentration of 2000 ppm) for applications such as CO2 capture from confined spaces like spacecraft, aircraft, and submarines, and by using the commercial adsorbent zeolite 13X. The CO2 purity achieved after each step of the process, together with the total amount of CO2 and N2 species, is presented in Fig. 1. This novel process yields higher CO2 purities than the ones obtained by standard previously used 5-step TVSA processes.
The performance of this process is expected to be further improved by coupling molecular modelling with process optimization. By doing so, we aim to screen the large pool of available MOF materials to find the best performing ones for this particular application. This analysis provides new insights into which relevant metrics need to be included when ranking materials for ultra-dilute gas separations, and identifies the top performing ones given the relevant process metrics.
Acknowledgement
The authors are thankful for the support from the PrISMa Project (No 299659), which is funded through the ACT programme (Accelerating CCS Technologies, Horizon2020 Project No 294766). Financial contributions made from: BEIS together with extra funding from NERC and EPSRC, UK; RCN, Norway; SFOE, Switzerland and US-DOE, USA, are gratefully acknowledged.
References
Boyd, P.G., Chidambaram, A., GarcÃa-DÃez, E., Ireland, C.P., Daff, T.D., Bounds, R., GÅ‚adysiak, A., Schouwink, P., Moosavi, S.M., Maroto-Valer, M.M., Reimer, J.A., Navarro, J.A.R., Woo, T.K., Garcia, S., Stylianou, K.C., Smit, B., 2019. Data-driven design of metal–organic frameworks for wet flue gas CO2 capture. Nature 576, 253–256.
Joss, L., Gazzani, M., Hefti, M., Marx, D., Mazzotti, M., 2015. Temperature swing adsorption for the recovery of the heavy component: An equilibrium-based shortcut model. Ind. Eng. Chem. Res. 54, 3027–3038.
National Academies of Sciences, Engineering, and Medicine, 2019. A Research Agenda for Transforming Separation Science. National Academies Press, Washington, DC.
Santori, G., Charalambous, C., Ferrari, M.C., Brandani, S., 2018. Adsorption artificial tree for atmospheric carbon dioxide capture, purification and compression. Energy 162, 1158–1168.
Sholl, D.S., Lively, R.P., 2016. Seven chemical separations to change the world. Nature 532, 435–437.
Sircar, S., Golden, T.C.C., Rao, M.B.B., 1996. Activated carbon for gas separation and storage. Carbon N. Y. 34, 1–12.
Figure 1. CO2 purity and total amount of each species at the end of each step of the novel TVSA processes, for removing CO2 from confined spaces (CO2 concentration of 2000 ppm) by using the commercial zeolite 13X. The system can reach 74% purity and 83% recovery by consuming 11.5 MJthermal/kgCO2. Main process parameters: 10 °C adsorption temperature, 100 °C desorption temperature, 1 kPa vacuum pressure level.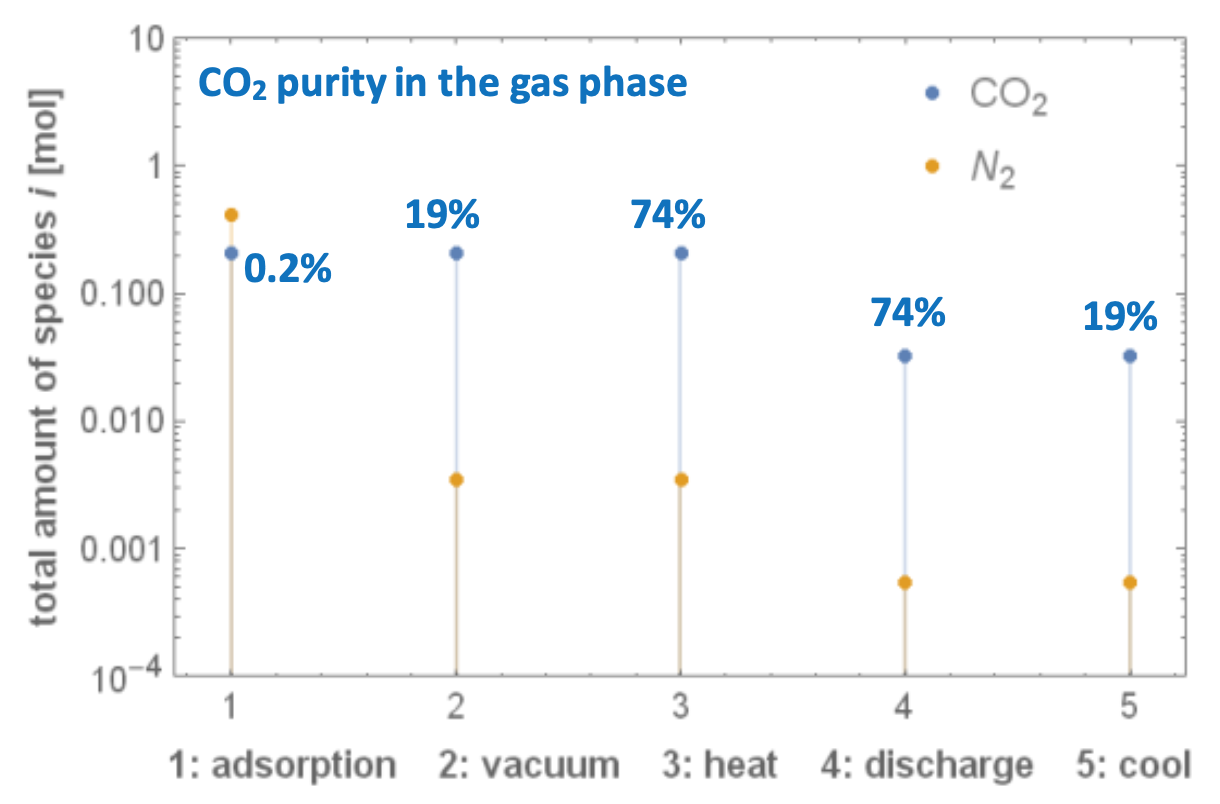
Topics
Checkout
This paper has an Extended Abstract file available; you must purchase the conference proceedings to access it.
Do you already own this?
Log In for instructions on accessing this content.
Pricing
Individuals
| AIChE Pro Members | $150.00 |
| AIChE Emeritus Members | $105.00 |
| AIChE Graduate Student Members | Free |
| AIChE Undergraduate Student Members | Free |
| AIChE Explorer Members | $225.00 |
| Non-Members | $225.00 |
