The Design Institute for Physical Properties (DIPPR) provides a software tool that facilitates use of the properties and prediction/calculation methods defined below. To obtain access, check out DIPPR’s pricing and ordering options.
- Acentric Factor (ACEN) - The acentric factor is defined as : ACEN = -log10(VP(0.7*TC)/PC) - 1.000000.
- Ambrose Method - Applies to Critical Temperature, Critical Pressure, Critical Volume. Reference: Ambrose, D., "Correlation and Estimation of Vapour-Liquid Critical Properties: I. Critical Temperatures of Organic Compounds, " Natl. Phys. Lab. Report Chem. 92, Middlesex, United Kingdom (1978).
- Autoignition Temperature (AIT) - The minimum temperature for a substance to initiate self-combustion in air leading to thermal runaway and a visible flame in the absence of an ignition source such as a spark or piloting flame, per some set of conditions.
- Baroncini Method - Applies to Liquid Thermal Conductivity. Reference: Baroncini, C., DiFilippo, F., Latini, G., Pacetti, M., "Organic Liquid Thermal Conductivity: A Prediction Method in the Reduced Temperature Range 0.3 to 0.8, " Int. J. Thermophys., 2, 1, 21 (1981).
- Benson Method - Applies to Ideal Gas Heat of Formation, Ideal Gas Heat Capacity. Reference: The ASTM Computer Program for Chemical Thermodynamic and Energy Release Evaluation. CHETAH 11.0, Users' Guide, ASTM International, PA (2020).
- Bhethanabotla Method - Applies to Liquid Viscosity. Reference: Bhethanabotla, V.R., "A Group Contribution Method For Liquid Viscosity, " M.S. Thesis, The Pennsylvania State University, University Park, PA (1983).
- Bondi Method - Applies to Heat of Fusion, Van der Waals Volume and Area. Reference: Bondi, A., "Physical Properties of Molecular Crystals, Liquids, and Glasses, " John Wiley, New York (1968).
- Brock-Bird Method - Applies to Surface Tension. Reference: Brock, J.R., Bird, R.B., AICHE J., 1, 174 (1955).
- BYU-DC Method - Applies to Dielectric Constant. Reference: Liu, J-P, Wilding, W. V., Giles, N. F., Rowley, R. L., "A Quantitative Structure Property Relation Correlation of the Dielectric Constant for Organic Chemicals, " J. Chem. Eng. Data, 55, 1, 41-45 (2010).
- BYU-NBP Method - Applies to Normal Boiling Point. Reference: Erickson, D., Wilding, W.V., Oscarson, J.L., Rowley, R.L., "Use of the DIPPR Database for Development of QSPR Correlations: Normal Boiling Point, " J. Chem. Eng. Data, 47, 1293-1302 (2002).
- BYU Flash Point Methods - Applies to Flash Point. Reference: Rowley, J.R., Rowley, R.L., Wilding, W.V., "Prediction of pure-component flash points for organic compounds, " Fire Mater., 35, 343-351 (2011) and Rowley, J.R., Rowley, R.L., Wilding, W.V., "Estimation of the Flash Point of Pure Organic Chemicals from Structural Contributions, " Process Saf. Prog., 29, 4, 353-358 (2010).
- BYU-FLVL Method - Applies to Flammability Limits. Reference: Rowley, J.R., Rowley, R.L., Wilding, W.V., "Estimation of the Lower Flammability Limit of Organic Compounds as a Function of Temperature, " J. Hazard. Mater., 186, 1, 551-557 (2011).
- BYU-FLVU Method - Applies to Flammability Limits. Reference: Rowley, J.R., "Flammability Limits, Flash Points, and Their Consanguinity: Critical Analysis, Experimental Exploration, and Prediction, Ph.D. Dissertation, " Brigham Young University (2010).
- BYU-HSUB Method - Applies to heat of Sublimation. Reference: Goodman, B. T., Wilding, W. V., Oscarson, J. L., Rowley, R. L., "Use of the DIPPR Database for the Development of QSPR Correlations: Solid Vapor Pressure and Heat of Sublimation of Organic Compounds, " Int. J. Thermophys., 25, 2, 337-350 (2004).
- BYU-Power law method and BYU-MDCP method - Applies to Solid Heat Capacity. Reference: Goodman, B. T., Wilding, W. V., Oscarson, J. L., Rowley, R. L., "Use of the DIPPR database for Development of Quantitative Structure-Property Relationship Correlations: Heat Capacity of Solid Organic Compounds, " J. Chem. Eng. Data, 49, 1, 24-31 (2004).
- BYU-Riedel Method - Applies to Liquid Vapor Pressure. Reference: Hogge, J.W., Giles, N.F., Rowley, R.L., Knotts, T.A., IV, Wilding, W.V., "New Vapor-Pressure Prediction with Improved Thermodynamic Consistency using the Riedel Equation, " Ind. Eng. Chem. Res., 56, 49, 14678-14685 (2017).
- BYU-SDN Method - Applies to Solid Density. Reference: Goodman, B. T., Wilding, W. V., Oscarson, J. L., Rowley, R. L., "A Note on the Relationship between Organic Solid Density and Liquid Density at the Triple Point, " J. Chem. Eng. Data, 49, 6, 1512-1514 (2004).
- BYU-SVP Method - Applies to Solid Vapor Pressure. Reference: Goodman, B. T., Wilding, W. V., Oscarson, J. L., Rowley, R. L., "Use of the DIPPR Database for the Development of QSPR Correlations: Solid Vapor Pressure and Heat of Sublimation of Organic Compounds, " Int. J. Thermophys., 25, 2, 337-350 (2004).
- BYU-TPL Method - Applies to Parachor. Reference: Knotts, T.A., Wilding, W.V., Oscarson, J.L., Rowley, R.L., "Use of the DIPPR Database for Development of QSPR Correlations: Surface Tension, " J. Chem. Eng. Data, 46, 1007-1012 (2001).
- Chickos Method - Applies to Heat of Fusion. Reference: Chickos, J.S., Braton, C.M., Hesse, D.G., Liebman, J.F., "Estimating Entropies and Enthalpies of Fusion of Organic Compounds, " J. Org. Chem., 56, 3, 927 (1991).
- Chung Method - Applies to Vapor Thermal Conductivity, Vapor Viscosity. Reference: Chung, T.H., Lee, L.L., Starling, K.E., "Applications of Kinetic Gas Theories and Multiparameter Correlation for Prediction of Dilute Gas Viscosity and Thermal Conductivity, " Ind. Eng. Chem. Fundam., 23, 8-13 (1984).
- Critical Compressibility Factor (ZC) - The value of the compressibility factor at the critical temperature. The calucation is defined as ZC = (PC * VC)*(R * TC)-1 where R is the Molar Gas Constant.
- Critical Pressure (PC) - The minimum pressure required for liquefaction of a gas at the critical temperature.
- Critical Temperature (TC) - The temperature above which a gas cannot be liquefied.
- Critical Volume (VC) - Molar volume of the substance at its critical point.
- Constantinou Method - Applies to Melting Point Temperature, Normal Boiling Point. Reference: Constantinou, L., Gani, R., "New Group Contribution Method for Estimating Properties of Pure Compounds, " AIChE J., 40, 10, 1697 (1994).
- Clapeyron Equation- Applies to Heat of Vaporization. Reference: Clapeyron, E.J., J. Ec. Polytech., 14, 23, 153 (1834).
- Clausius-Mossotti Equation and Onsager Equation- Applies to Dielectric Constant. Reference: Smyth, "Dielectric Behavior and Structure, " McGraw-Hill (1955).
- Dielectric Constant (DC) - Ratio of the electric field strength in vacuum to that in the material for the same charge distribution. Equivalently, it is the ratio of the capacitance between two parallel charged plates when filled with the material to that of a vacuum with identical charges on the plates. The value is reported at the reference temperature and pressure given by the following rules:
TC > 298.15 K and TPT < 298.15 K: T = 298.15 K, P = max[VP(298.15 K), 101325 Pa]
TC < 298.15 K: T = NBP, P = 101325 Pa
TPT > 298.15 K: T = TPT, P = TPP
An accepted experimental value must be for the liquid. - Dipole Moment (DM) - The first moment of the electric charge density expansion of a molecule.
- Density, Liquid (LDN) - Number of moles of a substance per unit volume in the liquid state.
- Density, Solid (SDN) - Number of moles of substance per unit volume in the solid state.
- Domalski and Hearing Method - Applies to Ideal Gas Heat of Formation, Ideal Gas Absolute Entropy, Standard State Heat of Formation, Standard State Absolute Entropy, Solid Heat Capacity. Reference: Domalski, E. S., Hearing, E. D., "Estimation of the Thermodynamic Properties of C-H-N-O-S-Halogen Compounds at 298.15 K, " J. Phys. Chem. Ref. Data, 22, 4, 805 (1993).
- Fedors Method - Applies to Critical Volume. Reference: Fedors, R. F., "A Method to Estimate Critical Volumes, " AIChE J., 25, 202 (1979).
- Flammability Limits (FLVL, FLVU) - The minimum/maximum concentration in air that will support flame propagation at the reference temperature given by the following rules:
FLTL < 273.15 K: Assumed to be 298.15 K, unless otherwise noted
FLTL > 273.15 K: Assumed to be FLTL/FLTU, unless otherwise noted. - Flammability Temperature Limits (FLTL, FLTU) - The lowest and highest temperatures, corrected to 101.3 kPa, at which the vapor/air mixture will support flame propagation.
- Flash Point (FP) - Lowest temperature, corrected to a pressure of 101325 Pa, at which application of an ignition source causes the vapors of a specimen in air to ignite under specified conditions of test.
- Freons Method - Applies to Normal Boiling Point. Reference: Shigaki, Y., Orimo, H., Fukushima, M., Mukai, Y., Ototake, N., "Estimation of the Basic Properties (Tb, Tc, Pc and W) of Fluorocarbon Refrigerants (Freons), " Int. Chem. Eng., 28, 3, 447 (1988).
- Govender Method - Applies to Liquid Thermal Conductivity. Reference: Govender, O., Rarey, J., Ramjugernath, D., "Estimation of Pure Component Properties, Part 5: Estimation of the Thermal Conductivity of Nonelectrolyte Organic Liquids via Group Contributions, " J. Chem. Eng. Data, 65, 3, 1300-1312 (2020).
- Harrison and Seaton Method - Applies to Ideal Gas Heat Capacity. Reference: Harrison, B.K., Seaton, W.H., "Solution to Missing Group Problem for Estimation of Ideal Gas Heat Capacities, " Ind. Eng. Chem. Res., 27, 1536-1540 (1988).
- Heat Capacity, Ideal Gas (ICP) - The amount of energy required to change the temperature of a unit mole of vapor one degree when the vapor is ideal; i.e., there are no intermolecular interactions.
- Heat Capacity, Liquid (LCP) - The amount of energy required to change the temperature of a unit mole of liquid one degree at constant pressure.
- Heat Capacity, Solid (SCP) - The amount of energy required to change the temperature of a unit mole of solid one degree.
- Heat of Fusion (HFUS) - The molar change in enthalpy when the solid is isothermally converted into a liquid at its melting point.
- Heat of Sublimation (HSUB) - The molar change in enthalpy when the solid is isothermally converted into a gas at its triple point temperature.
- Heat of Vaporization (HVP) - Difference between the enthalpies of a unit mole of a saturated vapor and saturated liquid of a pure component at any temperature and corresponding vapor pressure.
- Henry’s Law Constant of the Chemical in Water (HLC)- Property calculated from Henry's law constant, the partial fugacity of the chemical in solution in which the solvent is water and the mole fraction in the liquid phase:
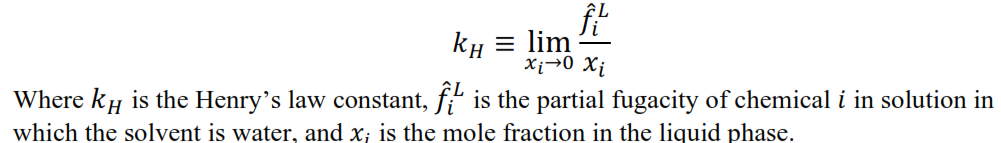
- Hsu Method - Applies to Liquid Viscosity. Reference: Hsu, H.-C., Sheu, Y.-W., Tu, C.-H., "Viscosity estimation at low temperatures (Tr < 0.75) for organic liquids from group contributions, " Chem. Eng. J., 88, 27-35 (2002).
- Ideal Gas Absolute Entropy (ENT) - Absolute entropy of the ideal gas at 298.15 K and 1 bar.
- Ideal Gas Gibbs Energy of Formation (GFOR) - The change in Gibbs energy associated with the reaction of forming the given chemical in the ideal gas state at 298.15 K and 1 bar from the elements in their standard states. The standard state for the elements is the stable phase at 298.15 K and 1 bar.
- Ideal Gas Heat of Formation (HFOR) - The enthalpy change associated with the formation reaction of the given chemical in the ideal gas state at 298.15 K from the elements in their standard states. The standard state for the elements is the stable phase at 298.15 K and 1 bar.
- Infinite Dilution Activity Coefficient of the Chemical in Water (ACCW) - Property calculated from the infinite dilution activity coefficient of the chemical, the partial fugacity of the chemical in solution in which the solvent is water, the pure liquid fugacity and the mole fraction in the liquid phase:

- Ishiuchi Method - Applies to Flash Point. Reference: Ishiuchi, Y., "Prediction of Flash Points of Flammable Liquids, " Anzen Kogaku, 15, 382-386 (1976).
- Joback Method - Applies to Critical Temperature, Critical Pressure, Ideal Gas Heat Capacity. Reference: Joback, K.G., "M.S. Thesis in Chemical Engineering, " Massachusetts Institute of Technology, Cambridge, MA (June 1984).
- Kopp’s method - Applies to Solid Heat Capacity. Reference: Hurst, J.E. Jr., Harrison, B.K., "Estimation of Liquid and Solid Heat Capacities Using a Modified Kopp's Rule, " Chem. Eng. Commun., 112, 21 (1992).
- Kubaschewski Method - Applies to Solid Heat Capacity. Reference: Kubaschewski, O., Unal, H., "An empirical estimation of the heat capacities of inorganic compounds, " High Temp. - High Pressures, 9, 361-365 (1977).
- Lee-Kesler Method - Applies to Heat of Vaporization, Liquid Vapor Pressure. Reference: Lee, B.I., Kesler, M.G., "A Generalized Thermodynamic Correlation Based on Three-Parameter Corresponding States, " AIChE J., 21, 3, 510 (1975).
- Leslie-Geniesse Method - Applies to Flash Point. Reference: Leslie, E.H., Geniesse, J.C., "International Critical Tables, vol. 2, " McGraw-Hill, 161 (1927).
- Liquid Molar Volume (LVOL) - Molar volume of the liquid at a reference temperature and pressure. The reference T and P are assigned according to the following rules:
TC > 298.15 K and TPT < 298.15 K: T = 298.15 K, P = max[VP(298.15 K), 101325 Pa]
TC < 298.15 K: T = NBP, P = 101325 Pa
TPT > 298.15 K: T = TPT, P = TPP - Lydersen Method - Applies to Critical Temperature, Critical Pressure, Critical Volume, Normal Boiling Point. Reference: Lydersen, A.L., "Estimation of Critical Properties of Organic Compounds, " University of Wisconsin Coll. Eng. Exp. Stn. Rep. 3, Madison, Wis. (April 1955).
- Maxwell and Bonnell - Applies to Liquid Vapor Pressure. Reference: Maxwell, J.B., Bonnell, L.S., "Vapor Pressure Charts for Petroleum Engineers, " Esso Research and Engineering Company, Linden, New Jersey (1955).
- McCann Method - Applies to Second Virial Coefficient. Reference: McCann, D.W., "A Group Contribution Method for Second Virial Coefficients, " M.S. Thesis, The Pennsylvania State University, University Park, Pennsylvania (1982).
- Meissner Method - Applies to Normal Boiling Point. Reference: Meissner, H.P., "Critical Constants from Parachor and Molar Refraction, " Chem. Eng. Progr., 45, 149 (1949).
- Melting Point Temperature (MP) - Temperature at which melting occurs at atmospheric pressure.
- Miller Method - Applies to Normal Boiling Point. Reference: Lyman, W.J., Reehl, W.F., Rosenblatt, D.H., "Handbook of Chemical Property Estimation Methods, " McGraw-Hill Book Co., New York (1982).
- Misic and Thodos Method - Applies to Vapor Thermal Conductivity. Reference: Misic, D., Thodos, G., "The Thermal Conductivity of Hydrocarbon Gases at Normal Pressures, " AIChE J., 7, 264 (1961).
- Modified Missenard Method - Applies to Liquid Thermal Conductivity. Reference: Missenard, A., "Conductivite Thermique des Solides, Liquides, Gaz et de Leurs Melanges, " Editions Eyrolles, Paris, 5 (1965); Also see Missenard, A., Comptes Rendus, 260, 5521 (1965).
- Molecular Weight (MW) - Molecular weight of the chemical as determined from the sum of the IUPAC atomic weights. It is used to convert specific properties to molar properties.
- Mostafa and Eakman Method - Applies to Standard State Heat of Formation, Standard State Gibbs Energy Heat of Formation, Standard State Absolute Entropy, Solid Heat Capacity. Reference: Golam Mostafa, A.T.M., Eakman, J.M., "Prediction of Standard Heats and Gibbs Free Energies of Formation of Solid Inorganic Salts from Group Contributions, " Ind. Eng. Chem. Res., 34, 4577 (1995).
- Myers Method - Applies to Parachor, Surface Tension, Liquid Thermal Conductivity. Reference: Myers, K.H., Danner, R.P., "Prediction of Properties of Silicon Boron and Aluminum Compounds, " J. Chem. Eng. Data, 38, 175 (1993).
- Nannoolal Method - Applies to Critical Temperature, Critical Pressure, Critical Volume, Normal Boiling Point, Liquid Viscosity. Reference: Nannoolal, Y., Rarey, J., Ramjugernath, D., "Estimation of pure component properties Part 2. Estimation of critical property data by group contribution, " Fluid Phase Equilib., 252, 1-27 (2007) and Nannoolal, Y., Rarey, J., Ramjugernath, D., "Estimation of pure component properties. Part 4: Estimation of the saturated liquid viscosity of non-electrolyte organic compounds via group contributions and group interactions, " Fluid Phase Equilib., 281, 97-119 (2009).
- Normal Boiling Point (NBP) - Temperature at which the liquid vapor pressure equals 101325 Pa.
- Othmer and Yu Method - Applies to Liquid Vapor Pressure. Reference: Othmer, D.F., Yu, E., "Correlating Vapor Pressures and Vapor Volumes, " Ind. Eng. Chem., 60, 1, 22 (1968).
- Pachaiyappan Method - Applies to Liquid Thermal Conductivity. Reference: Daubert, T.E., Danner, R.P., "Technical Data Book - Petroleum Refining, 6th Ed., " American Petroleum Institute, Washington, D.C. (1997).
- Pailhes Method - Applies to Normal Boiling Point. Reference: Pailhes, F., "Estimation of the Boiling Temperature at Normal Pressure for Organic Compounds from their Chemical Formula and a Known Boiling Temperature at Low Pressure, " Fluid Phase Equilib., 41, 97 (1988).
- Parachor (PAR) - Property calculated from the surface tension.
- Pintar Method - Applies to Autoignition Temperature. Reference: Pintar, A.J., "Estimation of Autoignition Temperature, " Technical Support Document, DIPPR Project 912, Michigan Technological University, Houghton, MI (July 1996).
- Przezdziecki and Sridhar Method - Applies to Liquid Viscosity. Reference: Przezdziecki, J.W., Sridhar, T., "Prediction of Liquid Viscosities, " AIChE J., 31, 333 (1985).
- Rackett Method - Applies to Liquid Density. Reference: Spencer, C.F., Danner, R.P., "Improved Equation for Prediction of Saturated Liquid Density, " J. Chem. Eng. Data, 17, 236 (1972).
- Radius of Gyration (RG) - Defined in terms of the principal moments of inertia (A,B,C) for a molecule as:

- Refractive Index (RI)- Ratio of the speed of light in a vacuum to the speed of light in the substance. The incident light is the sodium D line (0.5896 microns). The value is reported at the reference temperature and pressure given by the following rules:
TC > 298.15 K and TPT < 298.15 K: T = 298.15 K, P = max[VP(298.15 K), 101325 Pa]
TC < 298.15 K: T = NBP, P = 101325 Pa
TPT > 298.15 K: T = TPT, P = TPP
An accepted experimental value must be for the liquid. - Reichenberg Method - Applies to Vapor Viscosity. Reference: Reichenberg, D., "The Viscosity of Organic Vapors at Low Pressures, " DSC Rep. 11, National Physical Laboratory, Teddington, England (August 1971); Also see Reichenberg, D., AIChE J., 19, 854 (1973) and Reichenberg, D., AIChE J., 21, 181 (1975).
- Riedel Method - Applies to Liquid Vapor Pressure. Reference: Riedel, L., "Eine neue universelle Dampfdruckformel. Untersuchungen uber eine Erweiterung des Theorems der ubereinstimmenden Zustande. Teil I, " Chem. Ing. Tech., 26, 2, 83-89 (1954).
- Ruzicka and Domalski Method - Applies to Liquid Heat Capacity. Reference: Ruzicka, V., Domalski, E.S., "Estimation of the Heat Capacities of Organic Liquids as a Function of Temperature Using Group Additivity. 1. Hydrocarbon Compounds, " J. Phys. Chem. Ref. Data, 22, 3, 597 (1993) and Ruzicka, V., Domalski, E.S., "Estimation of the Heat Capacities of Organic Liquids as a Function of Temperature Using Group Additivity. II. Compound of Carbon, Hydrogen, Halogens, Nitrogen, Oxygen, and Sulfur, " J. Phys. Chem. Ref. Data, 22, 3, 619 (1993).
- Sastri-Rao Method - Applies to Liquid Thermal Conductivity, Liquid Viscosity. Reference: Sastri, S.R.S., Rao, K. K., "A new temperature-thermal conductivity relationship for predicting saturated liquid thermal conductivity, " J. Chem. Eng. Data, 74, 161-169 (1999) and Sastri, S. R. S., Rao, K. K., "A new group contribution method for predicting viscosity of organic liquids, " Chem. Eng. J., 50, 1, 9-25 (1992).
- Seaton Method - Applies to Flammability Limits. Reference: Seaton, W.H., "Group Contribution Method for Predicting the Lower and Upper Flammable Limits of Vapors in Air, " J. Hazard. Mater., 27, 169 (1991).
- Seaton Method - Applies to Autioignition Temperature. Reference: AITMP (tm) Version 94a. Autoignition Temperature Estimation Program for IBM PC/AT and Compatibles, Seadata (1994).
- Seaton-Redd-Guffey Method - Applies to Autoignition Temperature. Reference: Guffey, C.J., Redd, M.E., Giles, N.F., Knotts, T.A., IV, Wilding, W.V., "Radical isomerization and volatility considerations for improved autoignition temperature prediction, " Fuel, 393, 135016 (2025).
- Seaton-Redd and Seaton-Redd2 Methods - Applies to Autoignition Temperature. Reference: Redd, M.E., Seaton, W.G., Giles, N.F., Knotts, T.A., IV, Wilding, W.V., "An improved method for predicting autoignition temperatures based on first principles, " Fuel, 323, 124245 (2022).
- Second Virial Coefficient (SVR) - The first correction factor B from ideal gas in the virial expansion of the compressibility factor; i.e., Z= 1+B/V + ...
- Shebeko Methods - Applies to Flammability Limits. Reference: Shebeko, Yu.N., Ivanov, A.V., Dmitrieva, T.M., "Methods of Calculation of Lower Concentration Limits of Combustion of Gases and Vapors in Air, " Sov. Chem. Ind., 15, 3, 311-314 (1983) and Danner, R.P., Daubert, T.E., "Manual for Predicting Chemical Process Design Data, " AIChE, New York, NY (1990).
- Silva and Hall Method - Applies to Second Virial Coefficient. Reference: Iglesias-Silva, G. A., Hall, K. R., "An Equation for the Prediction and/or Correlation of Second Virial Coefficients, " Ind. Eng. Chem. Res., 40, 1968-1974 (2001).
- Solubility of the Chemical in Water (SOLW)- The maximum amount, in mole fraction, of a substance (gas, liquid, or solid) that will dissolve in water at the defined temperature and pressure.
- Solubility Parameter (SOLP) - Defined as
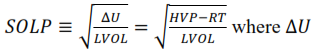
is the energy required to isothermally vaporize the liquid to the ideal gas state at a reference temperature and pressure given by the following rules:
TC > 298.15 K and TPT < 298.15 K: T = 298.15 K, P = max[VP(298.15 K), 101325 Pa]
TC < 298.15 K: T = NBP, P = 101325 Pa
TPT > 298.15 K: T = TPT, P = TPP - Standard State Absolute Entropy (SSTD) - Absolute entropy of the given chemical in the standard state. The standard state is defined as 298.15 K, 1 bar, and the stable phase at these conditions.
- Standard Net Heat of Combustion (HCOM) - The increase in enthalpy when a substance in its standard state at 298.15 K and 1 bar undergoes oxidation to defined combustion products. These combustion products are CO2 (g), H2O (g), F2 (g), Cl2 (g), Br2 (g), I2 (g), SO2 (g), N2 (g), P4O10 (cr), SiO2 (cristobalite), and Al2O3 (crystal, alpha).
- Standard State Gibbs Energy of Formation (GSTD) - The change in Gibbs energy associated with the reaction of forming the given chemical in its standard state from the elements in their standard states. The standard state in both cases is the stable phase at 298.15 K and 1 bar.
- Standard State Heat of Formation (HSTD) - The change in enthalpy associated with the reaction forming the given chemical in its standard state from the elements in their standard states. The standard state in both cases is the stable phase at 298.15 K and 1 bar.
- Stiel-Thodos Method - Applies to Vapor Thermal Conductivity, Vapor Viscosity. Reference: Stiel, L.I., Thodos, G., "The Thermal Conductivity of Nonpolar Substances in the Dense Gaseous And Liquid Regions, " AIChE J., 10, 26 (1964).
- Stein Method - Applies to Normal Boiling Point. Reference: Stein, S.E., Brown, R.L., "Estimation of Normal Boiling Point from Group Contributions, " J. Chem. Inf. Comput. Sci., 34, 3, 581 (1994).
- Sugden Method - Applies to Surface Tension. Reference: Sugden, S., "The Variation of Surface Tension with Temperature and Some Related Functions, " J. Chem. Soc., Trans., 125, 32 (1924).
- Surface Tension (ST) - The inherent force in the plane of the surface of a gas-liquid interface per unit length of surface which tends to minimize the surface area.
- Suzuki Method - Applies to Autoignition Temperature. Reference: Suzuki, T., Ohtaguchi, K., Koide, K., "Correlation and Prediction of Autoignition Temperatures of Hydrocarbons Using Molecular Properties, " J. Chem. Eng. Jpn., 25, 5, 606 (1992).
- Tarakad and Danner Method - Applies to Second Virial Coefficient. Reference: Tarakad, R.R., Danner, R.P., "An Improved Corresponding States Method for Polar Fluids: Correlation of Second Virial Coefficients, " AIChE J., 23, 685 (1977).
- Thermal Conductivity, Liquid (LTC) - The proportionality constant in Fourier’s law of heat conduction which describes the rate at which heat flows through a liquid.
- Thermal Conductivity, Solid (STC) - The proportionality constant in Fourier’s law of heat conduction which describes the rate at which heat flows through a solid.
- Thermal Conductivity, Vapor (VTC) - The proportionality constant in Fourier’s law of heat conduction which describes the rate at which heat flows through a vapor. The value is the ratio of energy flux per unit time divided by the temperature change per unit distance in the substance.
- Thomas Method - Applies to Liquid Viscosity. Reference: Thomas, L.H., "The Dependence of the Viscosities of Liquids on Reduced Temperature, and a Relation between Viscosity, Density, and Chemical Constitution, " J. Chem. Soc., 573 (1946).
- Triple Point Pressure (TPP) - Pressure at which equilibrium exists between solid, liquid, and vapor.
- Triple Point Temperature (TPT) - Temperature at which solid, liquid, and vapor of the substance are all in equilibrium.
- Tsonopoulos Method - Applies to Second Virial Coefficient. Reference: Tsonopoulos, C., "Second Virial Coefficients of Water Pollutants, " AIChE J., 24, 1112 (1978).
- Van der Waals Volume (VDWV) and Van del Waals Area (VDWA) - The excluded volume and surface area of model molecules treated as hard-sphere beads separated by rigid bonds.
- Van Velzen Method - Applies to Liquid Viscosity. Reference: Van Velzen, D., Lopes, Cardozo, R., Langenkamp, H., "Liquid Viscosity and Chemical Constitution of Organic Compounds. A New Correlation and a Compilation of Literature Data, " EUR 4735e, Commission of the European Communities, Luxembourg (1972).
- Vapor Pressure, Liquid (VP) - Vaporization pressure for liquid-vapor equilibrium.
- Vapor Pressure, Solid (SVP) - Sublimation pressure for solid-vapor equilibrium.
- Vera Method - Applies to Van der Waals Volume and Area. Reference: Vera, J.H., Sayegh, S.G., Ratcliff, G.A., "A Quasi Lattice-Local Composition Model for the Excess Gibbs Free Energy of Liquid Mixtures, " Fluid Phase Equilib., 1, 113 (1977).
- Viscosity (absolute), Liquid (LVS) - The shear stress per unit area at any point in a confined Newtonian liquid fluid divided by the velocity gradient in the direction perpendicular to the direction of flow.
- Viscosity, Vapor (VVS) - The shear stress per unit area at any point in a confined Newtonian vapor divided by the velocity gradient in the direction perpendicular to the direction of flow.
- Vogel Method - Applies to Refractive Index. Reference: Lyman, W.J., Reehl, W.F., Rosenblatt, D.H., "Handbook of Chemical Property Estimation Methods, " McGraw-Hill Book Co., New York (1982).
- Watson Method - Applies to Heat of Vaporization. Reference: Watson, K.M., "Prediction of Critical Temperatures and Heats of Vaporization, " Ind. Eng. Chem., 23, 360 (1931).
- Wildman and Crippen Method - Applies to Refractive Index. Reference: Wildman, S. A., Crippen, G. M., "Prediction of Physicochemical Parameters by Atomic Contributions, " J. Chem. Inf Comput. Sci., 39, 868-873 (1999).
- Wilson-Jasperson Method - Applies to Critical Temperature, Critical Pressure. Reference: Wilson, G.M., Jasperson, L.V., "Critical Constants TC, PC, Estimation Based on Zero, First and Second Order Methods, " AIChE Spring Meeting, New Orleans, LA (1996).
- Yoon and Thodos Method - Applies to Vapor Viscosity. Reference: Yoon, P., Thodos, G., "Viscosity of Nonpolar Gaseous Mixtures at Normal Pressures, " AIChE J., 16, 300 (1970).
