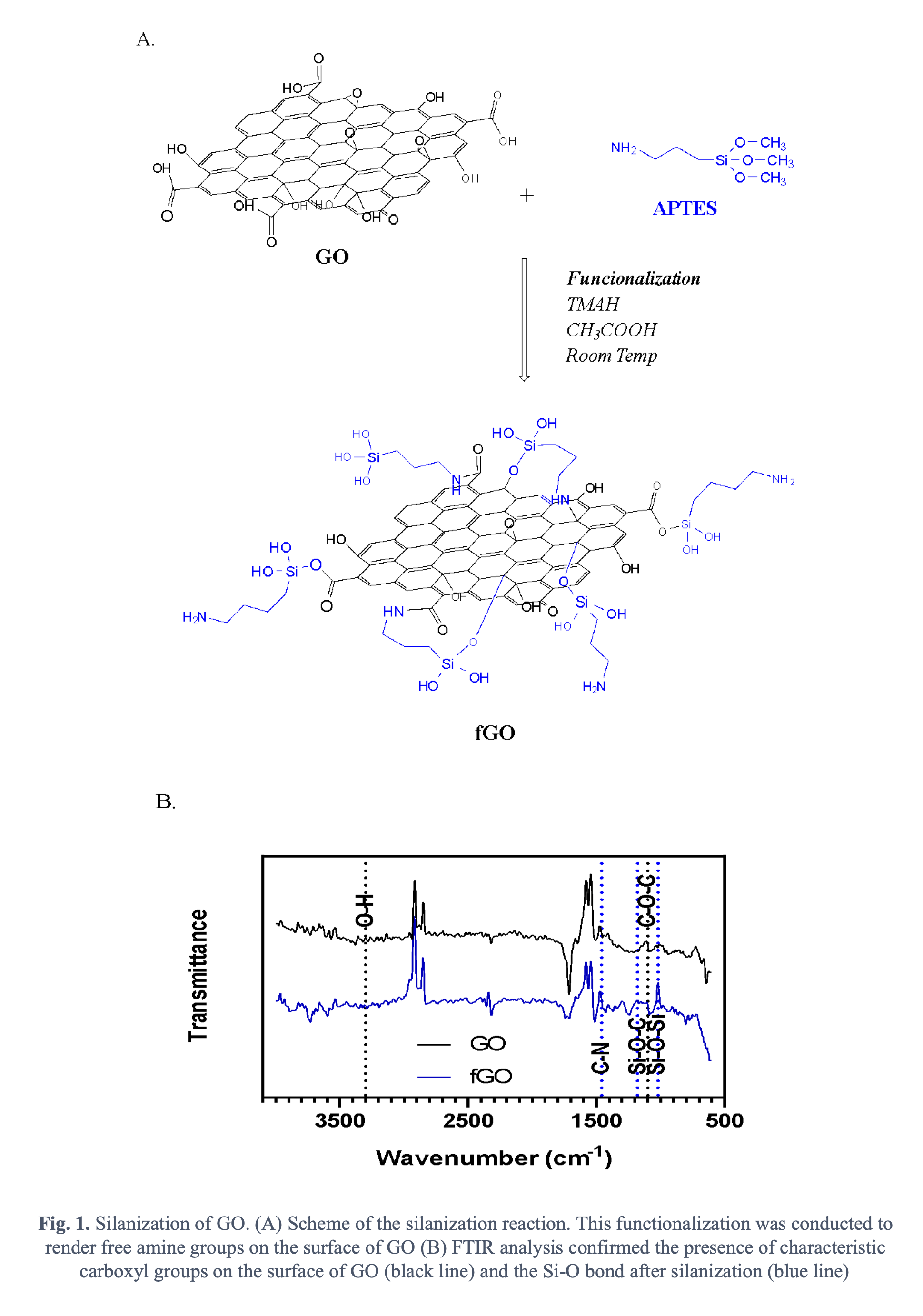
Accordingly, in this work we propose to put forward a new family of cell-penetrating based on GO-based nanoplatforms by interfacing GO with potent translocating peptides. This approach will take advantage of already tested and new peptides as well as the unique interactions of GO with the phospholipids of membranes and endosomes. Ideally, the platform should be able to carry a plethora of pharmacological agents but we are particularly interested in delivering gene therapies. GO was synthesized by the modified Hummers’ method through the oxidation of graphite sheets [6]. Briefly, 0.75 g of graphite sheets were mixed with 4.5 g of KMnO4 in a solution of H2SO4 (90 mL) and H3PO4 (10 mL). The mixture was left at 50°C and under continuous stirring at 350 RPM for 12 hours. Next, the mixture was cooled down at 0°C and H2O2 was subsequently added to quench the oxidation reaction. The resulting mixture was then exfoliated to obtain a solid that was centrifuged followed by resuspension in an aqueous solution of HCl and ethanol. Finally, the as-synthesized GO was filtered, lyophilized, and stored at 4ºC until further use. 100 mg of an aqueous suspension of GO were then silanized by adding 2 mL of tetramethylammonium hydroxide (TMAH) 25% (v/v), 50 µL of pure acetic acid and 1 mL of (3-Aminopropyl) triethoxysilane (APTES) 10% (v/v). A scheme of the reaction is shown in Fig 1A. The silanization was to render free amine groups on the surface of GO to further conjugate the peptide molecules. Thermogravimetric analysis (TGA) and Fourier-Transform Infrared spectroscopy were used to characterize the nanoplatform. FTIR analysis showed the distinctive peaks related to the characteristic carboxyl groups of GO (Fig 1B) as well as the Si-O bonds after silanization (Fig 1B). TGA allowed us to estimate a silanization efficiency of about 35%.
Future work will be focused on conjugating Buforin II and evaluating translocation efficiency by conducting uptake experiments in liposomes and various cell lines. Additionally, we will determine endosomal escape via confocal microscopy by labeling the peptide with fluorescent molecules and looking at colocalization with the fluorescent probe lysotracker.
This work provides an avenue for the development of gene delivery systems based on GO-based nanoplatforms. Moreover, by taking advantage of the great qualities in terms of physicochemical, electrical and optical properties of GO, this study might provide novel strategies to overcome limitations commonly faced such as low stability of the translocating biomolecules and endosomal entrapment.
References:
[1] N. Nayerossadat, P. Ali, and T. Maedeh, “Viral and nonviral delivery systems for gene delivery,†Adv. Biomed. Res., vol. 1, no. 1, p. 27, 2012.
[2] D. Ibraheem, A. Elaissari, and H. Fessi, “Gene therapy and DNA delivery systems,†Int. J. Pharm., vol. 459, no. 1–2, pp. 70–83, 2014.
[3] M. M. Mady, “Cationic liposomes as gene delivery system,†African J. Pharm. Pharmacol., vol. 5, no. 17, pp. 2007–2012, Nov. 2011.
[4] J. L. Shirley, Y. P. de Jong, C. Terhorst, and R. W. Herzog, “Immune Responses to Viral Gene Therapy Vectors,†Molecular Therapy, vol. 28, no. 3. Cell Press, pp. 709–722, 04-Mar-2020.
[5] M. Malmsten, “Inorganic nanomaterials as delivery systems for proteins, peptides, DNA, and siRNA,†Curr. Opin. Colloid Interface Sci., vol. 18, no. 5, pp. 468–480, 2013.
[6] N. D. Donahue, H. Acar, and S. Wilhelm, “Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine,†Adv. Drug Deliv. Rev., vol. 143, pp. 68–96, 2019.
[7] H. Hillaireau and P. Couvreur, “Nanocarriers’ entry into the cell: Relevance to drug delivery,†Cellular and Molecular Life Sciences, vol. 66, no. 17. Birkhauser Verlag Basel, pp. 2873–2896, 05-Jun-2009.
[8] E. Yuba et al., “PH-sensitive polymer-liposome-based antigen delivery systems potentiated with interferon-γ gene lipoplex for efficient cancer immunotherapy,†Biomaterials, vol. 67, pp. 214–224, Oct. 2015.
[9] A. Suarez-Arnedo et al., “Novel BUF2-magnetite nanobioconjugates with cell-penetrating abilities,†Int. J. Nanomedicine, vol. Volume 13, pp. 8087–8094, 2018.
[10] J. Sandoval et al., Carbon nanomaterials as pharmaceutic forms for sustained and controlled delivery systems. 2019.
[11] M. N. Hasan, M. Nafiujjaman, and Y.-K. Lee, 2D Nanomaterials for Gene Delivery. Elsevier Inc., 2019.
[12] N. Rahmanian, M. Eskandani, J. Barar, and Y. Omidi, “Recent trends in targeted therapy of cancer using graphene oxide-modified multifunctional nanomedicines,†Journal of Drug Targeting, vol. 25, no. 3. Taylor and Francis Ltd, pp. 202–215, 16-Mar-2017.
[13] D. de Melo-Diogo, R. Lima-Sousa, C. G. Alves, E. C. Costa, R. O. Louro, and I. J. Correia, “Functionalization of graphene family nanomaterials for application in cancer therapy,†Colloids Surfaces B Biointerfaces, vol. 171, no. May, pp. 260–275, 2018.
[14] P. Liu, S. Wang, X. Liu, J. Ding, and W. Zhou, “Platinated graphene oxide: A nanoplatform for efficient gene-chemo combination cancer therapy,†Eur. J. Pharm. Sci., vol. 121, no. March, pp. 319–329, 2018.