(649g) Nonlinear Model Predictive Control of an Integrated Continuous Purification System Using Pharmapy
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Pharmaceutical Discovery, Development and Manufacturing Forum
Pharma 4.0 (Advanced Controls, Process Automation, Data Analytics, etc.) - Processes and Applications
Thursday, November 9, 2023 - 5:42pm to 6:04pm
One challenge associated with implementing advanced control strategies in continuous purification systems is the complexity of the crystallization step, which is known to be significantly affected by process uncertainties (Nagy & Braatz, 2003). Thus, crystallization, filtrating, deliquoring, and drying must all be accurately modeled for a control strategy to efficiently reject the process disturbances and closely follow the setpoints (Destro et al., 2021). Also, the control strategy must accommodate changing conditions due to continued operation such as equipment fouling, and also uncertainty in thermophysical and kinetic parameters in order to optimize the performance of the physical system. In this regard, in silico studies enable the analysis of the effects of different control strategies using unit operation models, and also allow to compare their performance when applied on the integrated process as determined by achieved product quality, yield, and economic cost. In particular, first-principles unit operation models were shown to provide many advantages, including the fact that the models offer an accurate representation of the system dynamics by describing the underlying fundamental phenomena, allowing better prediction of response changes under different process inputs, and the possibility to be easily adapted to accommodate changing operating conditions, for example by updating the kinetics/thermophysical parameters to improve model prediction. Several novel control approaches have been proposed in the literature for incorporating robustness in the control of crystallization under uncertainties (Nagy & Braatz, 2003, Diehl et al., 2002), however there is a need to extend the framework to the overall integrated purification system, including crystallization, filtration and drying (Tosukhowong et al., 2004).
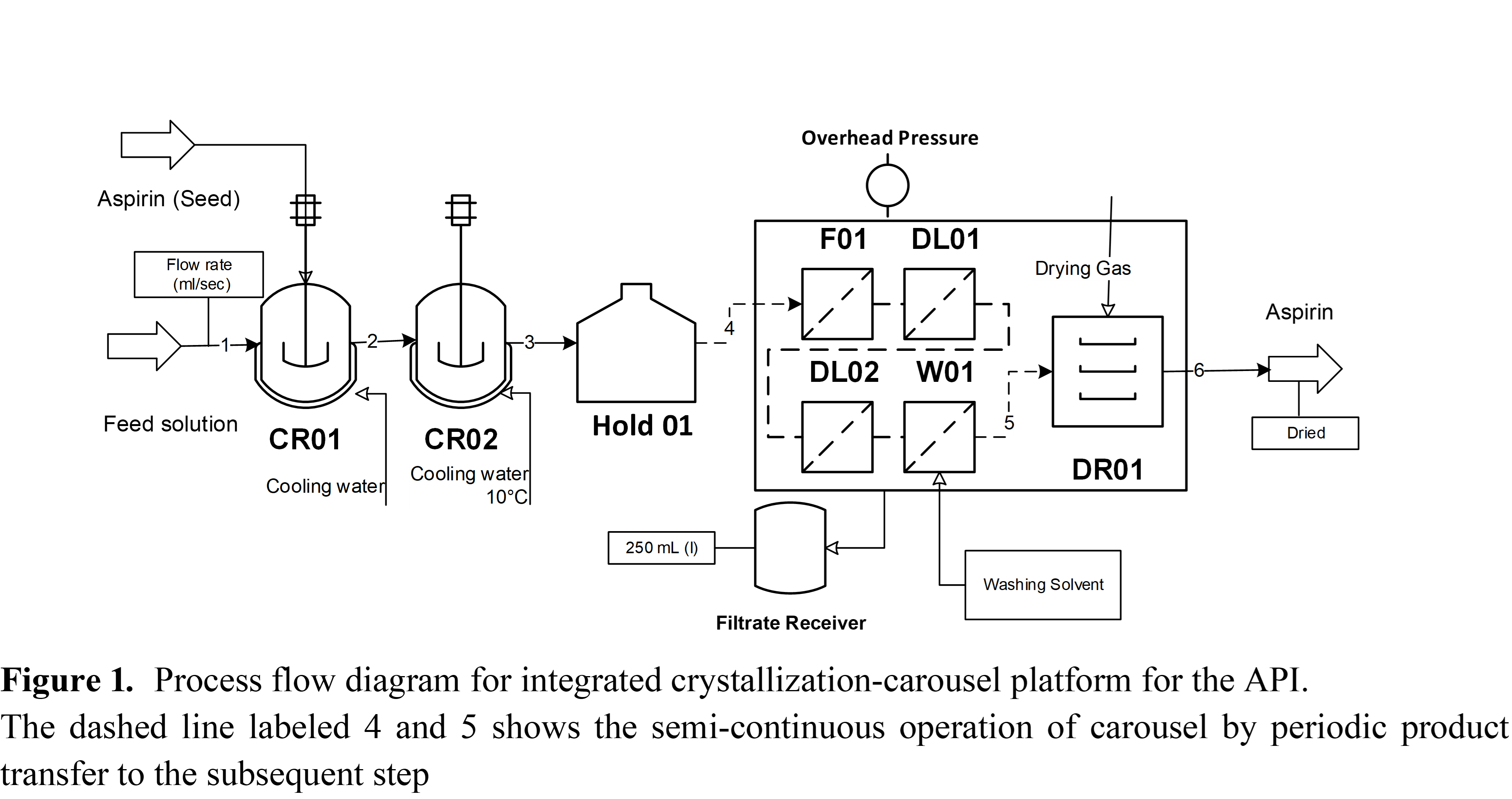
This work proposes an optimization-based control approach for the integrated API purification system using a digital twin of the process. The model is developed using the python-based modeling tool, PharmaPy, which aims to accurately predict the dynamic behavior of a continuous purification platform. The process flowsheet model is developed for the system that includes a two-stage continuous cooling crystallization cascade coupled with a carousel filter-dryer (an intensified semi-continuous filtration-deliquoring-washing-drying system), which mimics the operation of the integrated process unit developed by AWL (Alconbury Weston Ltd, UK) (Figure. 1).
Nonlinear model predictive control (NMPC) is implemented, which uses the flowsheet model for prediction and real-time optimization with the objective to maximize throughput while reducing filtration time and producing crystals of a required minimum size without generating unnecessarily large crystals. First, the digital twin was utilized to find the optimal process design as a result of the tradeoff between mean size (for better filterability) and maximum throughput via a multi-objective optimization framework. Different operation scenarios depending on the production rate were introduced during the continuous operation simulation. A diffusive horizon NMPC algorithm was implemented to control the flow rate and the CQAs of the crystalline products while also ensuring the feed rate allows the minimum cycle time required for filter-drying step to minimize a deviation of the process output trajectories from their targets. . The MHE takes measurements from the equipment and adjusts the uncertain parameters under changing process conditions, such as increasing fouling of the process and temperature fluctuation (Hur et al., 2022). The case study results demonstrate that the proposed control strategy leads to significantly reduced variability with improved product quality and resilience towards process disturbances. The in-silico investigations are supported by laboratory experiments. The results demonstrate the potential advantages of combining flowsheet simulation and efficient optimization frameworks for the advanced control of pharmaceutical manufacturing processes.
References
Pistikopoulos, E. N., Barbosa-Povoa, A., Lee, J. H., Misener, R., Mitsos, A., Reklaitis, G. V., Venkatasubramanian, V., You, F., & Gani, R. (2021). Process systems engineering – The generation next? Computers and Chemical Engineering, 147, 107252. https://doi.org/10.1016/j.compchemeng.2021.107252
Nagy, Z. K., & Braatz, R. D. (2003). Robust nonlinear model predictive control of batch processes. AIChE Journal, 49(7), 1776–1786. https://doi.org/10.1002/aic.690490715
Destro, F., Hur, I., Wang, V., Abdi, M., Feng, X., Wood, E., Coleman, S., Firth, P., Barton, A., Barolo, M., & Nagy, Z. K. (2021). Mathematical modeling and digital design of an intensified filtration-washing-drying unit for pharmaceutical continuous manufacturing. Chemical Engineering Science, 244, 116803. https://doi.org/10.1016/j.ces.2021.116803
Diehl, M., Bock, H. G., Schlöder, J. P., Findeisen, R., Nagy, Z., & Allgöwer, F. (2002). Real-time optimization and nonlinear model predictive control of processes governed by differential-algebraic equations. Journal of Process Control, 12(4), 577–585. https://doi.org/10.1016/S0959-1524(01)00023-3
Tosukhowong, T., Lee, J. M., Lee, J. H., & Lu, J. (2004). An introduction to a dynamic plant-wide optimization strategy for an integrated plant. Computers and Chemical Engineering, 29(1), 199–208. https://doi.org/10.1016/j.compchemeng.2004.07.028
Casas-Orozco, D., Laky, D., Wang, V., Abdi, M., Feng, X., Wood, E., Laird, C., Reklaitis, G. V., & Nagy, Z. K. (2021). PharmaPy: An object-oriented tool for the development of hybrid pharmaceutical flowsheets. Computers and Chemical Engineering, 153, 107408. https://doi.org/10.1016/j.compchemeng.2021.107408
Hur, I., Casas-Orozco, D., Reklaitis, G. V., & Nagy, Z. K. “Moisture content monitoring in continuous drug substance isolation manufacturing platformâ€, in AICHE Annual Meeting, 2022