(649f) Development of Digital Twins for API Synthesis Using Data-Rich Flow Experimentation
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Pharmaceutical Discovery, Development and Manufacturing Forum
Pharma 4.0 (Advanced Controls, Process Automation, Data Analytics, etc.) - Processes and Applications
Thursday, November 9, 2023 - 5:20pm to 5:42pm
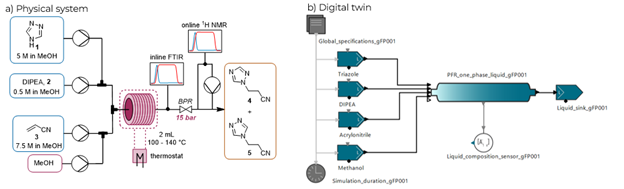
We highlight the development of a digital twin for a Michael addition continuous-flow process using data generated from automated dynamic flow experimentation (Fig. 1). gPROMS FormulatedProducts software was used for the creation of a digital twin. The dynamic flow experiments were fitted to a parallel reaction network for the reaction of 1,2,4-triazole with acrylonitrile in the presence of base to form the desired product and the isomer. The reaction network comprised of four kinetic parameters (A1, Ea1, A2 and Ea2). The fitted model closely corresponded to the experimental data, with R2 = 0.974. The residence time distribution (RTD) within the system was assessed using an automated step change protocol which examined the performance at different flow rates. We will demonstrate the utilization of the digital twin to explore the influence of disturbances within the system. We show that the digital twin can be used for the in-silico exploration of different manufacturing scenarios. The digital twin was experimentally validated using data form an autonomous self-optimization campaign.
Overall, we demonstrate that the development of digital twins in a pharma environment is easy to implement and flexible to the development constraints, and will thus facilitate early use of models for the identification of the sensitivity of product quality to parameter changes, significantly reducing development time and risks.