(296b) Optimal Control of Antibody Purification Via Protein a Affinity Chromatography during Multicycle Operations
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Computing and Systems Technology Division
Applied Math for Biomedical Systems
Wednesday, November 8, 2023 - 8:24am to 8:42am
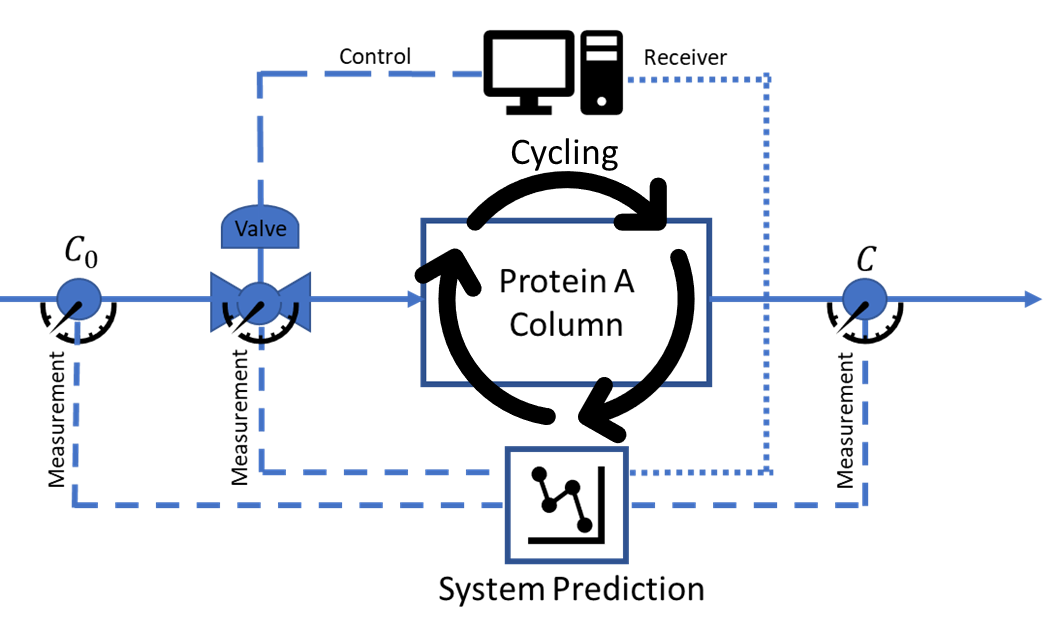
Monoclonal and polyclonal antibodies are essential therapies to treat many life-threatening diseases [1]. The most expensive step in antibody production is the Protein A chromatography resin used to selectively bind the antibody [2]. Many companies will use fixed residence time to maximize the antibody extraction capacity [3] and, as a result, minimize the amount of resin needed. Unfortunately, such strategy reduces the productivity of the purification step and increases the amount of water for injection needed. As a result, the use of fixed flow rates increases the overall costs of the operation and decreases the yield of the antibody due to the target molecule degradation with time [4].
Since the antibody yield and purity are improved at high flow rates but the resin capacity is improved at low flow rates, we investigate maximizing the resin performance by varying the flow rate while capturing the uncertainties of the process. Therefore, the objective of this work is to apply stochastic optimal control on a dynamic model, such as the Yoon-Nelson model [5], representing the affinity chromatography step, via inlet flow rate control to maximize the efficient use of the Protein A resin without sacrificing the yield and purity of the antibody. It is also expected that the resin will degrade with each cycle leading to a different optimal flow rate pathway. Therefore, the optimal control is expanded in our evaluation to also capture the resin performance during a multicycle process.
Methods:
Four temporal moments[6], are formulated to represent the dynamic chromatographic process model in the form of ordinary differential equations (ODEs). Stochastic optimal control is accomplished via the application of the Pontryagin’s maximum principle (PMP)[7] through the following steps: (i) integrate the Yoon-Nelson model with the method of moments[8] (ii) capture the uncertainty of the system via use of stochastic representations of the moments[9] (iii) determine the dynamic influent flow rate profile to achieve maximum antibody extraction10], and (iv) capture the multicycle degradation of the Protein A resin via linear representation and repeat the optimization steps. PMP also requires the introduction of deterministic and stochastic adjoint variables to complement the state variables, representing the four moments [10]. Resin performance optimization is achieved when the objective function, the theoretical plate number, is maximized at every time step.
Results:
When applying the Pontryagin’s maximum principle to maximize the resin performance, optimality was achieved via varying the flow rate with time. When the work is repeated with the same protein A resin for consecutive runs, the resin degradation was observed as the optimal flow rate pathway changed with each cycle enabling maximum resin lifetime usage.
Adding optimal control on the flow rate will allow the use of any size column via control of the residence time. Our work expands the efficient lifetime use of the most expensive step in the antibody downstream processing via maximizing yields, purity, and resin capacity in real life stochastic processes.
Keywords: Yoon-Nelson Model, stochastic optimal control, affinity chromatography.
References:
[1] R.-M. Lu et al., “Development of therapeutic antibodies for the treatment of diseases,†J. Biomed. Sci., vol. 27, no. 1, p. 1, Dec. 2020, doi: 10.1186/s12929-019-0592-z.
[2] S. Arora, B. V. Ayyar, and R. O’Kennedy, “Affinity Chromatography for Antibody Purification,†in Protein Downstream Processing, vol. 1129, N. E. Labrou, Ed. Totowa, NJ: Humana Press, 2014, pp. 497–516. doi: 10.1007/978-1-62703-977-2_35.
[3] A. M. Ramos-de-la-Peña, J. González-Valdez, and O. Aguilar, “Protein A chromatography: Challenges and progress in the purification of monoclonal antibodies,†J. Sep. Sci., vol. 42, no. 9, pp. 1816–1827, May 2019, doi: 10.1002/jssc.201800963.
[4] R. Molden et al., “Host cell protein profiling of commercial therapeutic protein drugs as a benchmark for monoclonal antibody-based therapeutic protein development,†mAbs, vol. 13, no. 1, p. 1955811, Jan. 2021, doi: 10.1080/19420862.2021.1955811.
[5] A. J. Shah, B. Soni, and S. K. Karmee, “Locally available agroresidues as potential sorbents: modelling, column studies and scale-up,†Bioresour. Bioprocess., vol. 8, no. 1, p. 34, Dec. 2021, doi: 10.1186/s40643-021-00387-1.
[6] J. W. Jawitz, “Moments of truncated continuous univariate distributions,†Adv. Water Resour., vol. 27, no. 3, pp. 269–281, Mar. 2004, doi: 10.1016/j.advwatres.2003.12.002.
[7] I. M. Ross, A primer on Pontryagin’s principle in optimal control. Carmel, Calif. : Collegiate Publishers, 2009., 2009. [Online]. Available: https://search.library.wisc.edu/catalog/9910894537702121
[8] M. N. Goltz and P. V. Roberts, “Using the method of moments to analyze three-dimensional diffusion-limited solute transport from temporal and spatial perspectives,†Water Resour. Res., vol. 23, no. 8, pp. 1575–1585, Aug. 1987, doi: 10.1029/WR023i008p01575.
[9] K. M. Yenkie and U. Diwekar, “Stochastic Optimal Control of Seeded Batch Crystallizer Applying the Ito Process,†Ind. Eng. Chem. Res., p. 120604103933002, Jun. 2012, doi: 10.1021/ie300491v.
[10] P. T. Benavides and U. Diwekar, “Studying various optimal control problems in biodiesel production in a batch reactor under uncertainty,†p. 9, 2013.