(116h) Analysis of Lipid Vesicles with Surface Acoustic Wave Stimulation
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Engineering Sciences and Fundamentals
Poster Session: Fluid Mechanics
Monday, November 6, 2023 - 3:30pm to 5:00pm
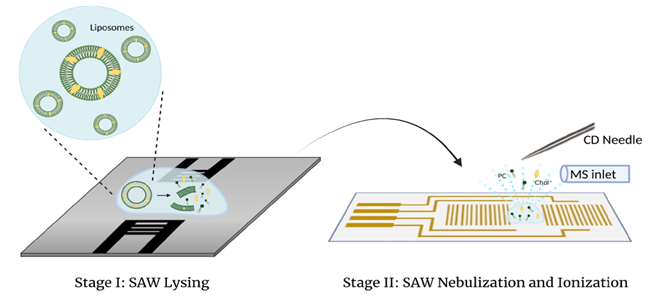
The characterization of liposome by using mass spectrometry (MS) offers several benefits. The molecule compositions inside liposome can be analyzed at very low concentration, and MS also can distinguish lipids and molecules with similar structures, making it much easier to identify and quantify specific components inside the liposome. However, some challenges can be encountered when doing direct MS analysis on liposomes, mainly due to their size variances and their tendency to form large clusters in gas and liquid form. Moreover, current sample preparation methods often include organic solvents and detergents to disrupt liposomes, which may cause protein denaturization and addition of substances into the sample. In this study, we investigate a mechanical lysis approach by using high frequency surface acoustic wave (SAW) to disrupt liposomes, which allows for direct MS analysis with corona discharge (CD) ionization. High frequencies SAW up to 100 MHz were firstly used to lyse DOPC liposome samples. Then the lysed sample was transferred to a standing wave chip and rapidly nebulized at higher SAW power. A commercial high voltage APCI needle were used for corona discharge to ionize non-polar analytes from nebulized samples. Results show that when subjected to higher SAW frequencies, DOPC liposomes experienced higher degree of phospholipid bilayer disruption by producing free DOPC signals monitored by MS. Lysing time also shows a direct correlation to DOPC lipid ion signal. SAW-lysed 1 μL DOPC samples were detectable at the concentration of 1.6 μM, which prove this sample preparation method can be advantageous in direct analysis for precious, low-volume vesicle samples.