(622c) Controlled Loading of Albumin-Drug Pre-Conjugates for Treatment of Pancreatic Cancer
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Nanoscale Science and Engineering Forum
Bionanotechnology for Gene and Drug Delivery II
Friday, November 20, 2020 - 8:30am to 8:45am
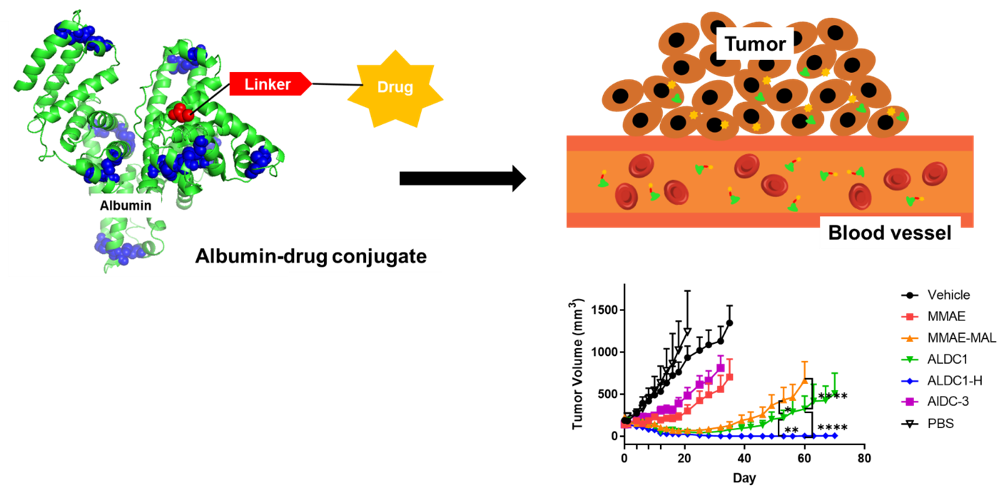
Chemotherapy drugs have been widely used as standard of care to treat cancers for several decades. However, due to their promiscuous cytotoxicity, they can cause adverse events and have narrow dosing regimens, which limit their efficacy. In part to address these challenges, drug delivery systems have been developed to improve the therapeutic index of existing chemotherapy drugs, including drug derivates, micelles, liposomes, and polymeric nanoparticles. However, it remains a challenge to achieve potent delivery of therapeutics in tumors without high levels of accumulation in non-specific tissues. Here, we developed albumin-drug conjugates with controlled loading to harness the intrinsic transport properties of albumin and improve the therapeutic index of current in situ albumin-binding prodrugs for significantly improved antitumor efficacy. Here, model drug monomethyl auristatin E (MMAE) was conjugated to Cys34 of albumin via a cathepsin B-sensitive dipeptide linker ex vivo to ensure that all drug would be bound specifically to albumin. The resulting albumin-drug conjugate with a drug to albumin ratio (DAR) of 1 (ALDC1) retained the native structure of albumin compared to conjugate with a higher DAR of 3 (ALDC3). ALDC1 exhibited improved drug release and cytotoxicity compared to ALDC3 in vitro. ALDC1 exhibited slower plasma clearance and increased drug exposure over time compared to ALDC3 and MMAE prodrug. In single dose studies with MIA PaCa2 xenografts, cohorts treated with ALDC1 had the highest amount of MMAE drug in tumor tissues compared to other treatment arms. After multiple dosing, ALDC1 significantly delayed the tumor growth compared to control treatment arms MMAE, MMAE-linker conjugate and ALDC3. When dosed with the maximum tolerated dose of ALDC1, there was complete eradication of 83.33% of the tumors in the treatment group. ALDC1 also significantly inhibited tumor growth in an immunocompetent syngeneic mouse model that recapitulates the phenotype and clinical features of human pancreatic cancers. In summary, site-specific loading of drug to albumin at 1:1 ratio allowed the conjugate to maintain the native structure of albumin and its intrinsic properties. By conjugating the drug to albumin prior to administration minimized premature cleavage and instability of the drug in plasma and enabled higher drug accumulation in tumors compared to in situ albumin-binding prodrugs. This strategy to control drug loading ex vivo ensures complete drug binding to the albumin carrier and achieves excellent antitumor efficacy, and it has the potential to greatly improve the outcomes of anticancer therapy.