(609a) Synergistic Effects of Graphene and Nanofibers Alignment on Muscle Regeneration
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Regenerative Engineering Society
Regenerative Engineering: Advanced Biomaterials for Complex Tissue Regeneration
Thursday, November 19, 2020 - 9:30am to 9:45am
Materials and Methods: The scaffolds were fabricated by electrospinning the composite solutions of pristine Graphene nanoplatelets (GnPs) and poly (l-lactic acid) (PLLA) with different amounts of GnPs include 0, 0.5, 1.5, and 2 mg/ml. The micromorphology of the nanofibers was examined via scanning electron microscopy (SEM). The nanofiber diameter, surface functional groups, mechanical properties, and electrical conductivity were evaluated by Image J software, Fourier Transform Infrared spectroscopy (FTIR), Instron machine, and four-point probe device, respectively. To investigate the muscle cells proliferation and differentiation, mouse C2C12 myoblasts were cultured on the scaffolds in standard GM and investigated through live/dead assay, CCK8 assay, and immunostaining with myosin heavy chain (MHC).
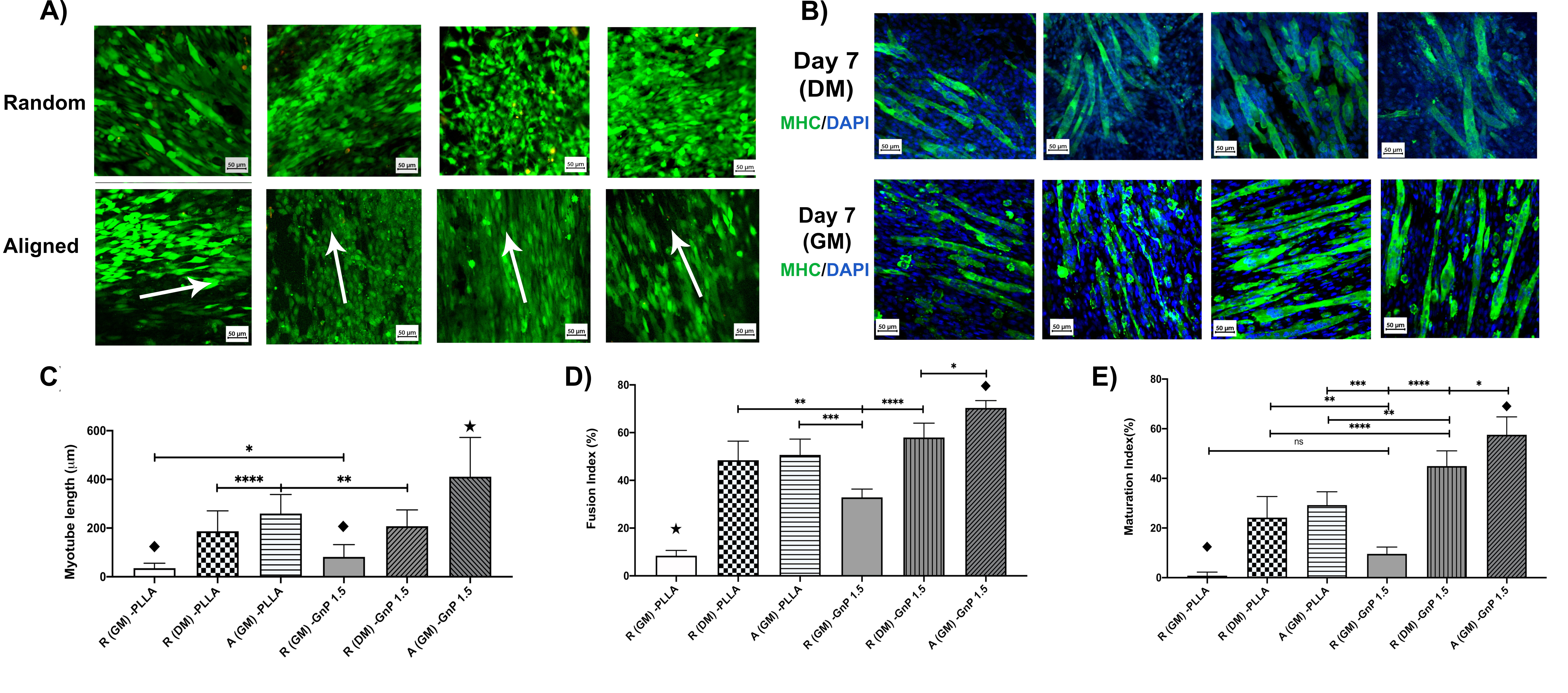
Results and Discussion: Based on the results, incorporation of GnPs in PLLA nanofibers significantly improved mechanical properties and enhanced electrical conductivity compared with pure PLLA scaffold while no significant difference was observed in the morphology of the scaffolds. Fig1.A shows that all concentrations of pristine GnPs promoted myoblasts growth and proliferation 7 days after cell culture. Moreover, the significant formation of myotubes on all the scaffolds in standard GM can be observed in Fig1.B. The statistical analysis demonstrated that topographical cue even in standard GM had the highest impact on the length of myotubes while electrical cue in biomimetic DM had the highest effects on myotube fusion and maturation. The combination of topographical cue and electrical cue demonstrated the highest differentiation and maturation grade on the formed myotubes in GM, suggesting that the guidance cues further promoted aligned, long, and multinucleated myotube formation. The myotube length, fusion, and maturation indices of A(GM)-GnP 1.5 were further increased to 411.4 ± 160.8 µm, 70.37 ± 3.044%, and 57.59 ± 7.163%, respectively (Fig 1.C-E). These results showed that the combination of topographical and electrical cues in standard GM was more efficient than a single cue alone in DM for stimulating myoblast differentiation and myotube maturation.
Figure 1. A) Live/dead fluorescent images of the random and aligned scaffolds after 7days (green=viable cells; red=dead cells), Arrows show the main directions of nanofibers alignment. B) Immunofluorescent images of differentiated myotubes on the random and aligned scaffolds after 7 days (MHC=green and nucleus=blue). Quantification of C) Myotube length, D) Fusion index, E) Maturation index, (* = P ≤ 0.05, ** = P ≤ 0.01, *** = P ≤ 0.001, **** = P ≤ 0.0001; n=5).
Conclusions: The study showed that a combination of electrical and topographical cues can significantly improve the physical properties of the scaffolds. Incorporation of GnPs as an electrical cue into highly aligned nanofibers significantly improved the myoblasts growth and proliferation and induced myotube formation in standard GM without the need for external electrical stimulation.
Acknowledgments: We acknowledge the financial support received from DHHS/NIH/ NIAMS entitled, Regenerative Engineering of Complex Musculoskeletal Tissue and Joints, Grant No.: DP1AR068147.