(439f) Asymmetric Alkylation Reaction at the Interface of Achiral Surfactant Assemblies
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Engineering Sciences and Fundamentals
Self-Assembly in Solution
Thursday, November 19, 2020 - 9:15am to 9:30am
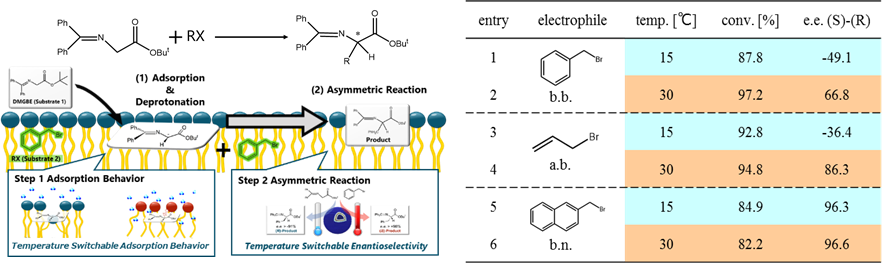
In this study, the distribution (adsorption) behavior of substrates onto DDAB membranes were studied to elucidate the reaction selectivity. First, DMGBE was added to the suspension of DDAB vesicles, and the UV spectrum was measured to indicate the state of substrate after interaction with vesicles. The peak around 250 nm, derived from DMGBE, shifted at 15 °C, suggesting that the deprotonated state (carbanion or enolate form) might be stable on vesicle. On the other hand, no peak shifts were observed at 30 °C. From these results, DMGBE could be differently adsorbed on DDAB membranes depending on temperature. The enantioselectivity of asymmetric reaction by changing the electrophile was investigated. The asymmetric reaction of DMGBE was performed on a suspension of DDAB vesicles using electrophiles. When b.n. was used, (S)-product was preferentially generated regardless of temperature, while, b.b. and a.b. resulted in the temperature-dependency on the product chirality. Although the membrane is constructed by achiral surfactants, the vesicle membrane can be act as the interface that control the localization of substrates, which provide a preferential interaction between reactants.