(365a) Electrically-Heatable Graphene Aerogels As Nanoparticle Supports in High-Pressure CO2 Capture
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Separations Division
CO2 Capture By Adsorption I
Tuesday, November 17, 2020 - 8:00am to 8:15am
Hydrotalcite-derived nanoparticles (HTs) are the most important group of CO2 adsorbents for SE-H2 since they show their highest CO2 sorption performance in the temperature (473-773 K) and pressure range of interest (1-30 bar). Under these conditions, CO2 physisorbents (e.g. zeolites, activated carbons) have low capacities and selectivities, while other potential CO2 chemisorbents (lithium zirconates and calcium oxides) exhibit slow adsorption kinetics or require high energy to be regenerated. Despite the positive sorption characteristics of HTs for SE-H2, their overall multicycle performance in terms of capacity, stability and energy efficiency needs to be further improved before they can be successfully used commercially. In this regard, it has been reported that significant enhancement in the CO2 adsorption performance of HTs can be obtained by dispersing them on high surface area materials such as carbons.[4]
In this work we address the main challenges of SE-H2 by supporting Mg-Al HT nanoparticles on 3D-reduced graphene oxide (rGO) aerogels, which are particularly suitable carbon structures due to the matching morphology and surface charge between the HT and the graphene support. For this purpose, unsupported Mg-Al HTs are synthesised via co-precipitation. To prepare HT/rGO supported aerogels, a well-dispersed GO suspension and a pre-fabricated wet-paste of HT are mixed. The aerogels are obtained by freeze drying and reduced with 5 % H2(N2) at 1273 K. Physicochemical characterisation techniques such as BET, TGA, XRD, TEM, SEM, XPS and state-of-the-art equipment including an Intelligent Gravimetric Analyser (IGA), are used to gain fundamental insights into adsorptive performance under realistic temperature and pressure conditions for SE-H2. Full details of the experimental methodology used are given elsewhere.[5]
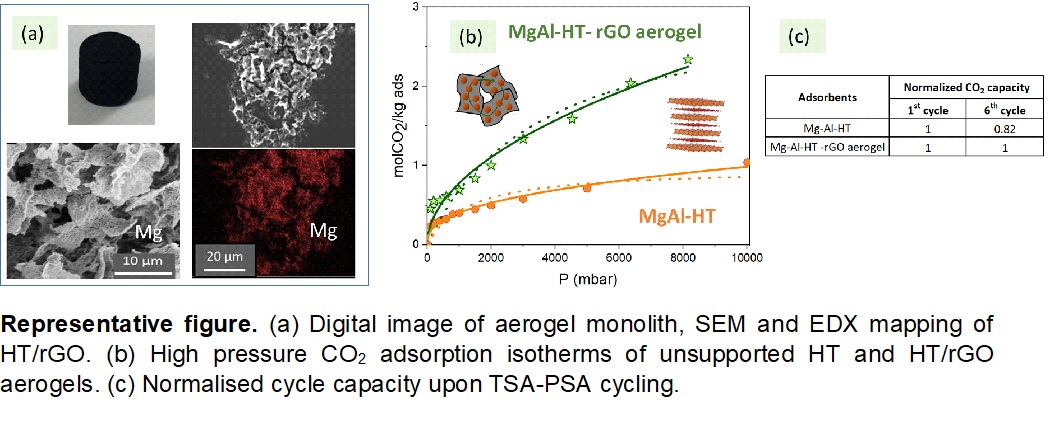
The results of the work show that hydrotalcite-derived Mg-Al nanoparticles are successfully supported on rGO aerogels at very high HT loadings (~80 wt %), which is needed for the design of compact and efficient sorption units. The HT/rGO aerogel hybrids exhibit significantly higher CO2 uptake compared to the unsupported HTs, showing an enhancement of ~230% at 573 K and PCO2 = 8 bar. Temperature-pressure swing (573-673 K, 10 mbar-10 bar) tests show that the initial capacity of the pure HT drops by 20-30% upon cycling while the uptake of the aerogel hybrids is reduced only by 1-5%. The substantial benefits obtained by the presence of the rGO aerogel in HTs are attributed to electrostatic compatibility, excellent electrical conductivity, and enhanced mesoporosity and particle dispersion. The novel HT/rGO aerogel hybrids markedly outperforms other HT-carbon supported systems (e.g. GO, rGO, carbon nanotubes and nanofibers), and other type of relevant materials (e.g. activated carbons, MOFs, metal oxides and zeolites) in terms of capacity and stability. Under the operating cyclic conditions used, the CO2 isotherms of the adsorbents can be modelled by the Freundlich equation, which is characteristic of heterogeneous surfaces.
In this contribution, we provide the first data on the CO2 adsorption performance of carbon supported Mg-Al HTs at both elevated temperature and partial pressures of CO2 relevant for industrial SE-H2. In addition, we present for the first time the use of electrically-conducting rGO aerogels as very efficient supports to enhance the CO2 adsorption properties of HTs. Research efforts are currently being devoted to extend this ground-breaking study by assessing the performance of the novel HT/rGO hybrids with other metal combinations such as Cu-Al and Ni-Al that have shown to be highly active and stable for the most relevant catalytic reactions involved in SE-H2. Emphasis is given to further investigate the mechanical properties and thermal stability of the materials for their use in fixed bed and fluidised bed reactors. In addition, the regeneration of the hybrids by temperature-pressure swing adsorption is complemented by Joule-heating analyses. The use of the Toth and the linear driving force models is explored as a source of useful information for the prediction of SE-H2 units. The feasibility of using this type of hybrids in other relevant H2-CCS intensified processes such as adsorptive desulfurization and chemical looping combustion (CLC) is also under investigation.
Acknowledgements
This work is supported by the ELEGANCY, EU H2020 project and the Imperial College London Research Fellowship Grant. The authors are grateful to Milo Shaffer and David Chadwick for discussions.
References
- IEAGHG report, Techno-economic evaluation of SMR based standalone hydrogen plant with CCS, (2017).
- Valdsund, K. Jordal and R. Anantharaman, “Hydrogen production with CO2 capture,†Int. J. Hydrog. Energy, 41, pp. 4969-4992 (2016).
- Iruretagoyena, K. Hellgardt, D. Chadwick, “Towards autothermal hydrogen production by sorption-enhanced water gas shift and methanol reforming: A thermodynamic analysisâ€, Int. J. Hydrogen Energy, 43, pp. 4211-4222 (2018).
- Iruretagoyena, Supported layered double hydroxides for sorption enhanced hydrogen production, Springer Theses, Switzerland, (2016).
- Menzel, D. Iruretagoyena et al., “Electrically-Heatable Graphene Aerogels as Nanoparticle Supports in Adsorptive Desulfurisation and High-Pressure CO2 Captureâ€, submitted.