(240c) Ultra-Deformable Liposomes for Enhanced Drug Delivery
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Food, Pharmaceutical & Bioengineering Division
Formulations of New Particulate Carriers
Wednesday, November 18, 2020 - 8:15am to 8:30am
Our lab has previously demonstrated that altering nanolipogel elasticity resulted in a Young’s modulus-dependent trend in cellular and tumor uptake. These data established that uptake of soft (< 1.6 MPa) were internalized by cells compared to their stiffer (> 13.8 MPa) counterparts [4]. One approach to modulating liposomal deformability is to alter the composition of the lipid bilayer. This can be achieved by incorporating lipids of different acyl chain lengths or degrees of saturation, as well as the use of edge activators (i.e. surfactants). Incorporation of phospholipids with short acyl chain length (less than 14) or multiple double bonds (poly-unsaturated), may disrupt the lipid bilayer and consequently increase fluidity [5]. It is hypothesized that hypo-elastic, ultra-deformable particles are internalized preferentially due to two unique capabilities: (1) internalization via fusion with the cell membrane (a process of low energy dependence) [4] and (2) squeezing through small pores to permeate tissues [6]. Novel formulations developed herein, termed ultra-deformable liposomes (UDLs) have demonstrated increased cancer cell internalization and transendothelial cell permeability.
Materials and Methods: UDLs were formulated with 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC; C12) or 1,2-diheptanoyl-sn-glycero-3-phosphocholine (DHPC; C7), and 1% Benzoxazolium, 3-octadecyl-2-[3-(3-octadecyl-2(3H)-benzoxazolylidene)-1-propenyl]-, perchlorate (DiO; a lipophilic carbocyanine dye, which fluoresces when incorporated into a lipid bilayer) for visualization. Liposomes were synthesized using the thin film hydration method. Liposomal size and polydispersity was determined via dynamic light scattering (DLS), using a Brookhaven Zeta-PALS analyzer (Brookhaven Instruments, Holtsville, NY, USA). To study the internalization of UDLs by TNBC cells, MDA-MB-436 or MDA-MB-231 cells were seeded at 75,000 per well in a 24 well plate and incubated overnight at 37C. Cells were treated with 200 nM UDLs for 2-4 hours at 37°C. Cells were rinsed twice with PBS prior to analysis with flow cytometry (Beckman Coulter, CytoFLEX). Quantification of liposome internalization by cells was determined by DiO fluorescence (ex/em: 484 nm), normalizing for background fluorescence. Transendothelial permeability studies were performed using human umbilical vein endothelial cells (HUVECs) seeded on transwell inserts (Corning) and incubated for 2 days prior to use. Then, 200 nM UDLs were added to the upper well of the insert and equal volume of DMEM was added to the bottom of the chamber. Transwells were incubated at 37°C for 4 hours then the bottom chamber media was removed and read on a spectrometer for DiO fluorescence.
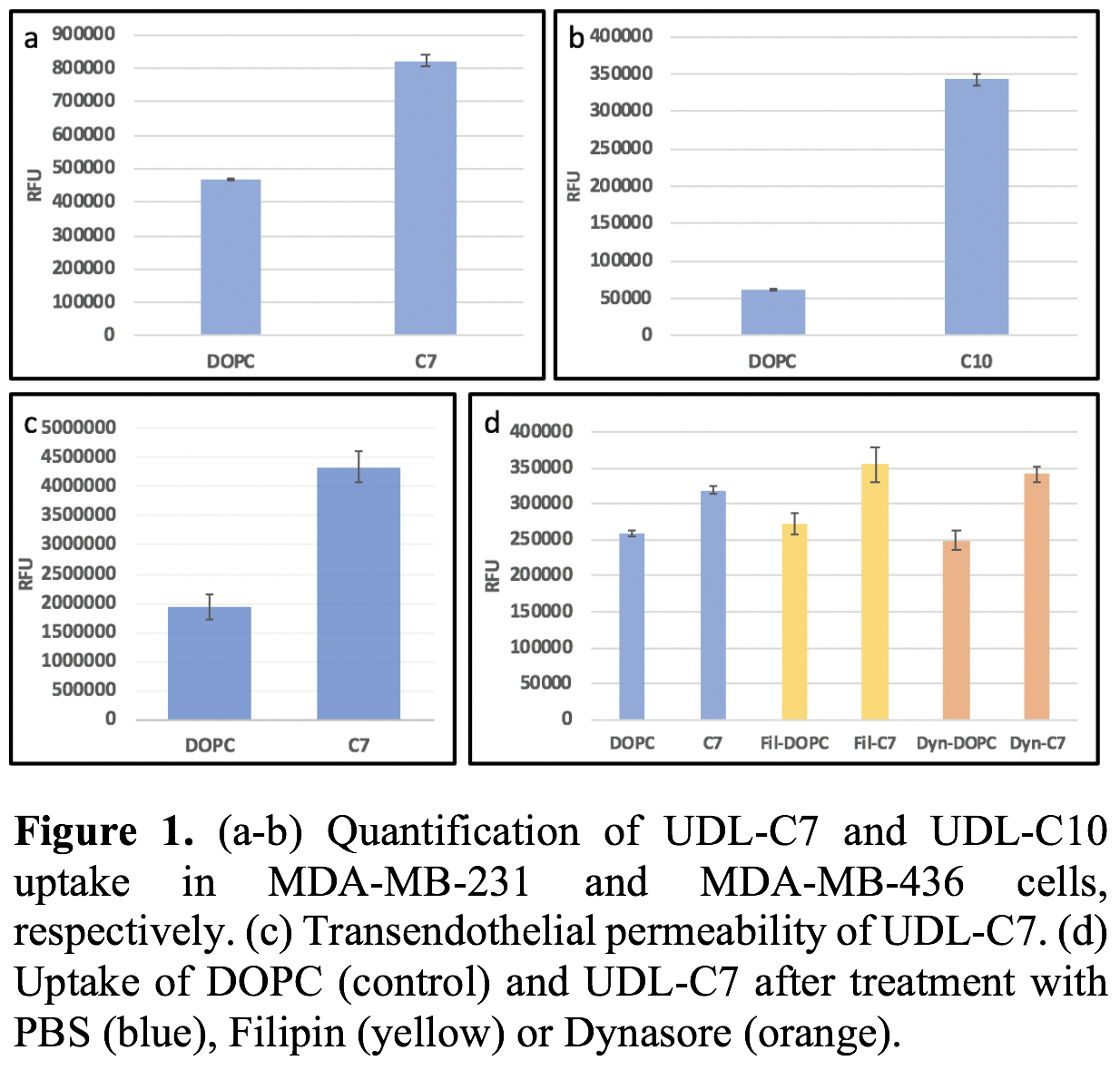
Results: Novel UDL formulations using a combination of DOPC and short chain phospholipids (C7 or C10) were prepared and characterized by size, zeta potential and Young’s moduli. UDL-C7 and UDL-C10 formulations demonstrated 1.7-fold and 5.4-fold increase in TNBC cell uptake relative to DOPC control liposomes, respectively (Fig 1a-b). Further, UDL-C7 demonstrated a 2.3-fold increase in transendothelial membrane permeation, compared to DOPC control (Fig 1c). Preliminary data on the inhibition of cell internalization pathways (clathrin or caveolae) show that UDL-C7 liposomes are internalized via fusion, and not receptor mediated endocytosis (Fig 1d). Moving forward, we will show that UDLs will increase accumulation within tumors in vivo.
Conclusions: The novel UDL formulations developed herein demonstrated increased internalization by TNBC cells and transendothelial permeability compared to DOPC control liposomes. Similar to our previous findings, these data show that UDL-C7 are internalized predominantly by fusion, not clathrin or caveolae mediated endocytosis.
References: [1] Barsky, S. H., Rao, C. N., Grotendorst, G. R., & Liotta, L. A. (1982). The American journal of pathology, 108(3), 276. [2] Takai, K., Le, A., Weaver, V. M., & Werb, Z. (2016). Oncotarget, 7(50), 82889–8290. [3] Stylianopoulos, T., et al. (2012). Proceedings of the National Academy of Sciences, 109(38), 15101-15108. [4] Guo, P., et al (2018). Nature communications, 9(1), 1-9. [5] Hussain, A., et al. (2017). International journal of nanomedicine, 12, 5087–5108. [6] Cevc, G., Schätzlein, A., & Richardsen, H. (2002). Biochimica et Biophysica Acta (BBA)-Biomembranes, 1564(1), 21-30.