(162r) Sonication-Free Fabrication and Characterization of Hydrogel-Embedded PLGA Microspheres for Extracellular Vesicle Delivery to the Intervertebral Disc
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Materials Engineering and Sciences Division
Poster Session: Materials Engineering & Sciences (08B - Biomaterials)
Thursday, November 19, 2020 - 8:00am to 9:00am
Back pain is a leading cause of global disability with a lifetime prevalence of up to 84% in adults.[1, 2] The total cost associated with lower back pain in the United States is 134.5 billion, indicating a great socioeconomic burden associated with the condition.[3] Back pain is strongly associated with degeneration of the intervertebral disc (IVD), whose primary function is biomechanical and allows for controlled motion and flexibility of the spinal column upon movement. The innermost layer of the IVD is the nucleus pulposus (NP), a jelly-like core composed of collagen and elastin fibers embedded in a highly hydrated gel.[4] Surrounding the NP is the annulus fibrosus (AF), made up of lamellae also containing collagen and elastin fibers. AF defects, often caused by disc herniation, are clinically challenging to repair. This is due to the avascular nature of the disc, which results in limited healing capacity.[5, 6] Healing typically involves the formation of fibrotic tissue, followed by the deposition of disorganized and collagenous matrix at the site of injury. This ultimately leads to fibrotic scarring and progressive IVD degeneration overtime.[6] Current treatment strategies for IVD degeneration, such as lumbar discectomy, are unsuccessful because they fail to address the poor healing capacity of the disc, as indicated by the prevalence of disc reherniation. Thus, there is a substantial unmet clinical need to develop therapeutic strategies that promote endogenous repair of the AF, maintain IVD function, and are able to withstand complex spinal loads.[7, 8]
Hydrogel technologies have emerged as promising strategies for IVD repair in that they can provide structural support to damaged tissues and allow for the incorporation of bioactive factors to enhance biological repair. A two-part repair strategy has been developed consisting of (1) an interpenetrating network (IPN) hydrogel composed of synthetic (poly(ethylene glycol) diacrylate/20 kDa PEGDA) and natural (fibronectin-conjugated Fibrin/FN-Fibrin) polymer networks, and (2) a dual-modified (oxidized and methacrylated) hyaluronic acid (HAMA Aldehyde) that covalently bonds this injectable hydrogel to IVD collagen.[9] While this two-part biomaterial system primarily serves to seal AF defects, the IPN also serves as a delivery vehicle for biologics to enhance the IVD’s innate limited healing capacity. Extracellular vesicles (EVs) were chosen as the biologic of interest due to their important role as messengers in intercellular communication under physiological conditions.[10] In order to control biologic release rate and establish a physiochemical barrier to preserve EV stability upon exposure to ammonium persulfate/N,N,N',N'-tetramethylethane-1,2-diamine (APS/TEMED), the redox-initiator pair used to crosslink the IPN-hydrogel, we propose the use of poly(lactic-co-glycolic acid) (PLGA) microspheres as a biomaterial carrier that can engraft within the IPN hydrogel system.[11] However, most reported single- and double-emulsion techniques require ultrasonication steps to fabricate PLGA microspheres, which may disrupt the integrity of the EV lipid bilayer membrane and potentially lead to a loss of its biological cargo.[10] Here, we report a sonication-free method to fabricate PLGA microspheres for the encapsulation of EVs and to minimize agitation of EV solutions. Furthermore, we report the engraftment of microspheres within the IPN hydrogel system for the controlled release of EVs to the IVD.
Methods
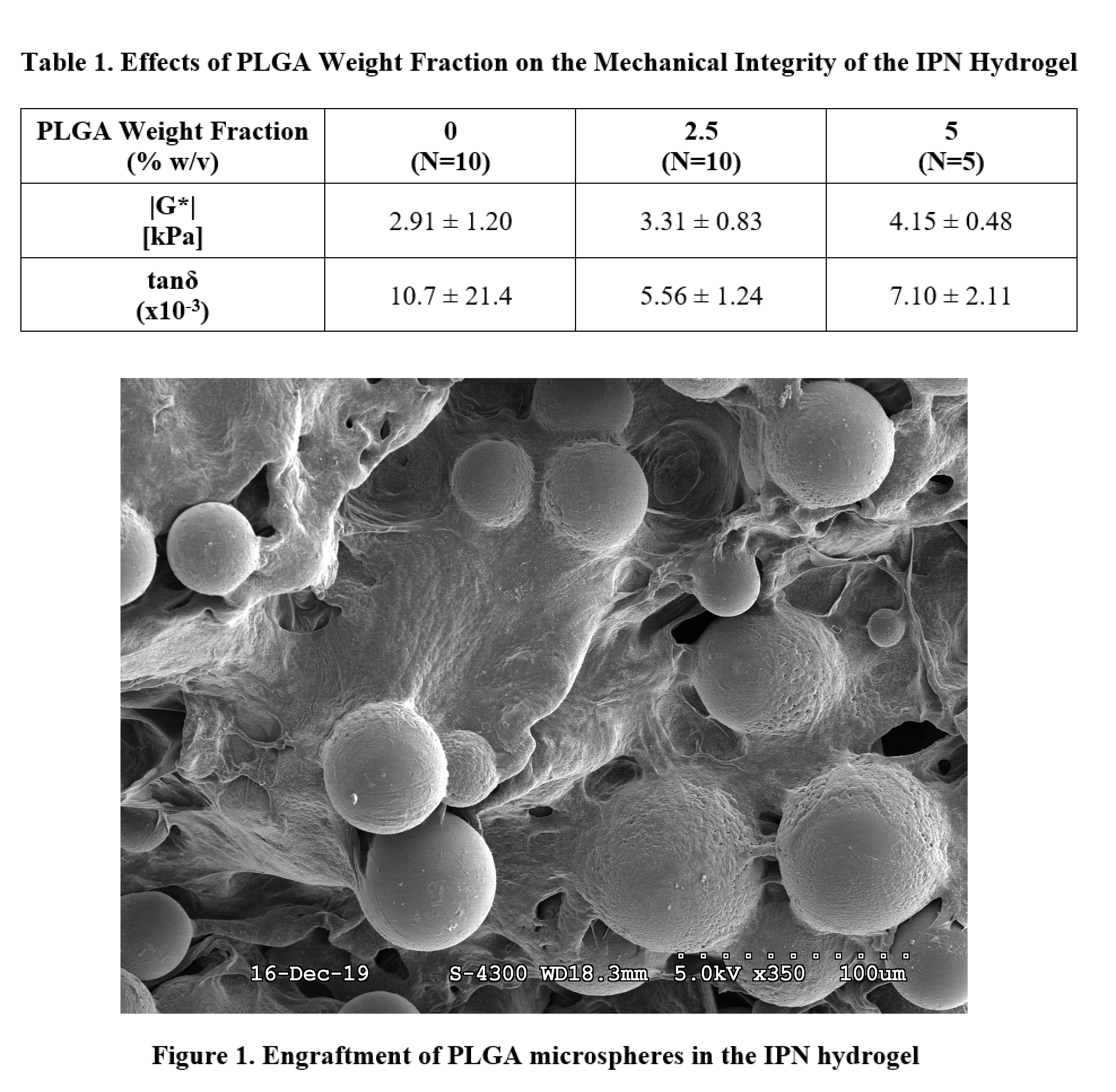
A sonication-free emulsion technique was performed to fabricate blank PLGA microspheres. The vortexing time of the solution consisting of the PLGA, chloroform, and poly(vinyl alcohol) was varied in four conditions: 15 s, 30 s, 45 s, and 60 s. Scanning electron microscopy (SEM) was performed to characterize the particle morphology and polydispersity across the four conditions. The 15 s fabrication condition was chosen for downstream applications. 20 kDa IPN hydrogels containing various mass fraction percentages of PLGA microspheres were fabricated in 8 mm diameter by 3 mm height cylindrical molds and polymerized via redox initiation with 20 mM APS/TEMED. IPN hydrogels incorporated 5 mg/mL FN-Fibrin and were crosslinked via thrombin-mediated polymerization.[12] The mass fraction percentages of PLGA microspheres in the IPN hydrogel was varied in three conditions: 0%, 2.5%, and 5% w/v PLGA (N=5-10/group). SEM imaging of hydrogels corresponding to each condition was performed. Identical cylindrical specimens underwent a torsional frequency sweep from 0.1 – 10 Hz in pure shear to measure the complex shear modulus (|G*|) and phase angle (tanδ), with results reported at 1 Hz. One-way ANOVA with Tukey’s post-hoc was used to assess significance.
Results and Discussion
This study investigates the effect of vortexing time on the morphology and polydispersity of blank PLGA microspheres, as well as the effect of microsphere incorporation on the mechanical integrity of the IPN hydrogel. The microsphere fabrication technique was optimized in order to minimize any mechanical destruction of EVs during the fabrication process, and was therefore performed sonication-free. SEM imaging of microspheres fabricated under the four vortexing conditions did not show any observable differences in particle morphology or polydispersity across the groups. Therefore, the minimum vortexing time of 15 s was chosen for downstream applications as it introduces the least amount of force on the EVs being encapsulated. Results of the mechanical testing on the microsphere-engrafted IPN hydrogels indicated that increasing the PLGA weight fraction from 0% to 5% w/v resulted in a slight increase in the complex shear modulus (Table 1). Furthermore, incorporating PLGA microspheres resulted in a slight decrease in the tangent phase angle. SEM imaging of the IPN hydrogels confirmed that microspheres were successfully engrafted and that the morphology of the microspheres was unchanged (Figure 1). Therefore, results of this proof of concept study indicate that the incorporation of engrafted microspheres in the hydrogel did not significantly impact the mechanical integrity of the system.
Due to concerns about the integrity of EVs in the presence of free radicals, PLGA microspheres are meant to serve as a transient layer of protection for EVs during the redox initiated polymerization of the IPN hydrogel. Additionally, the use of microsphere carriers for EVs is advantageous in that it can allow for better localization and concentration of EVs within annular defects in the IVD, potentially enhancing the IVD’s innate limited healing capacity.[13] While EVs were not encapsulated in microspheres in this study, the optimization of the microsphere fabrication technique is necessary for future studies in which EVs will be encapsulated. Furthermore, long-term degradation studies of the microsphere-engrafted IPN hydrogel will be performed to analyze how degradation of the microspheres affects the mechanical integrity of the hydrogel system.
Acknowledgements: Funded by NIH/NIAMS R01AR057397. Special thanks to The Lillian and Henry M. Stratton-Hans Popper Department of Pathology Microscopy Facility at The Icahn School of Medicine at Mt. Sinai.
References
[1] B.F. Walker, The Prevalence of Low Back Pain: A Systematic Review of the Literature from 1966 to 1998, Clinical Spine Surgery 13 (2000) 205-217.
[2] X. Yang, X. Li, Nucleus pulposus tissue engineering: a brief review, European Spine Journal 18 (2009) 1564.
[3] J.L. Dieleman, J. Cao, A. Chapin, C. Chen, Z. Li, A. Liu, C. Horst, A. Kaldjian, T. Matyasz, K.W. Scott, A.L. Bui, M. Campbell, H.C. Duber, A.C. Dunn, A.D. Flaxman, C. Fitzmaurice, M. Naghavi, N. Sadat, P. Shieh, E. Squires, K. Yeung, C.J.L. Murray, US Health Care Spending by Payer and Health Condition, 1996-2016, JAMA 323 (2020) 863-884.
[4] J.P.G. Urban, S. Roberts, Degeneration of the intervertebral disc, Arthritis Res Ther 5 (2003) 120-130.
[5] J.P. Urban, S. Holm, A. Maroudas, A. Nachemson, Nutrition of the intervertebral disc: effect of fluid flow on solute transport, Clin Orthop Relat Res (1982) 296-302.
[6] O. Torre, R. Das, R. Berenblum, A. Huang, J. Iatridis, Neonatal mouse intervertebral discs heal with restored function following herniation injury, The FASEB Journal 32 (2018) fj.201701492R.
[7] L.J. Smith, N.L. Nerurkar, K.-S. Choi, B.D. Harfe, D.M. Elliott, Degeneration and regeneration of the intervertebral disc: lessons from development, Dis Model Mech 4 (2011) 31-41.
[8] M. Likhitpanichkul, M. Dreischarf, S. Illien-Junger, B.A. Walter, T. Nukaga, R.G. Long, D. Sakai, A.C. Hecht, J.C. Iatridis, Fibrin-genipin adhesive hydrogel for annulus fibrosus repair: performance evaluation with large animal organ culture, in situ biomechanics, and in vivo degradation tests, Eur Cell Mater 28 (2014) 25-38.
[9] T.J. DiStefano, J.O. Shmukler, W.W. Hom, S.B. Nicoll, J.C. Iatridis, Evaluation of ex vivo herniation risk of a novel two-part strategy for annulus fibrosus repair, Orthopedic Research Society Annual Meeting, Phoenix, AZ, 2020.
[10] S.G. Antimisiaris, S. Mourtas, A. Marazioti, Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery, Pharmaceutics 10 (2018) 218.
[11] M.R. Battig, Y. Huang, N. Chen, Y. Wang, Aptamer-functionalized superporous hydrogels for sequestration and release of growth factors regulated via molecular recognition, Biomaterials 35 (2014) 8040-8048.
[12] T.J. DiStefano, D. Goldberg, S.B. Nicoll, J.C. Iatridis, Tissue integration of acrylate-based hydrogels via multifunctional chondroitin sulfate for annulus fibrosus repair, Orthopedic Research Society Annual Meeting, Austin, TX, 2019.
[13] X. Liu, Y. Yang, Y. Li, X. Niu, B. Zhao, Y. Wang, C. Bao, Z. Xie, Q. Lin, L. Zhu, Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration, Nanoscale 9 (2017) 4430-4438.