(9d) Demonstration of a Sustainable Pathway for Producing Fully Bio-Based Polyethylene Terephthalate (bio-PET)
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Process Development Division
Environmentally Friendly Product and Process Development for Sustainability
Monday, November 6, 2023 - 9:15am to 9:40am
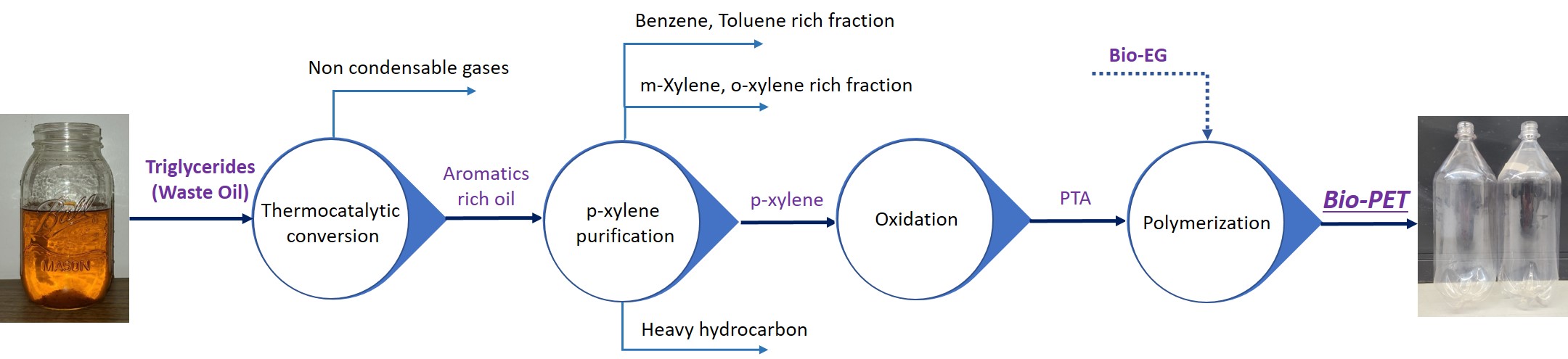
Our innovation for thermo-catalytic conversion of waste oil involves delivery of the feed into a hot reactor via an atomizer to allow the oils to vaporize rapidly and facilitate catalytic reactions in the vapor phase. As a result, our pyrolysis reactor generates products with high yields, high selectivity, and low coke formation on the catalyst. At the optimum reactor temperature of 500°C and a weight hourly space velocity of 6 h-1, we have measured organic liquid product yields of 63% (relative to feed mass) with an aromatics content of 48%. Further, we have shown in-situ catalyst regeneration and reuse for 10 reaction runs without measurable impact on product yield; the duration of each run was 30 min. The product spectrum is similar to the mixture obtained from commercial petrochemical production and allows for easy p-xylene separation. We will present data on upstream conversion, downstream purification, and polymer synthesis to demonstrate a fully viable pathway for bio-PET by our process (see Figure below). Property comparisons of bio-PET and petro-PET will also be presented.