(98e) Development and Evaluation of Novel Biphasic Solvents for Post-Combustion Carbon Capture
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Sustainable Engineering Forum
CO2 Capture for Industrial Point Sources
Sunday, November 5, 2023 - 4:42pm to 5:00pm
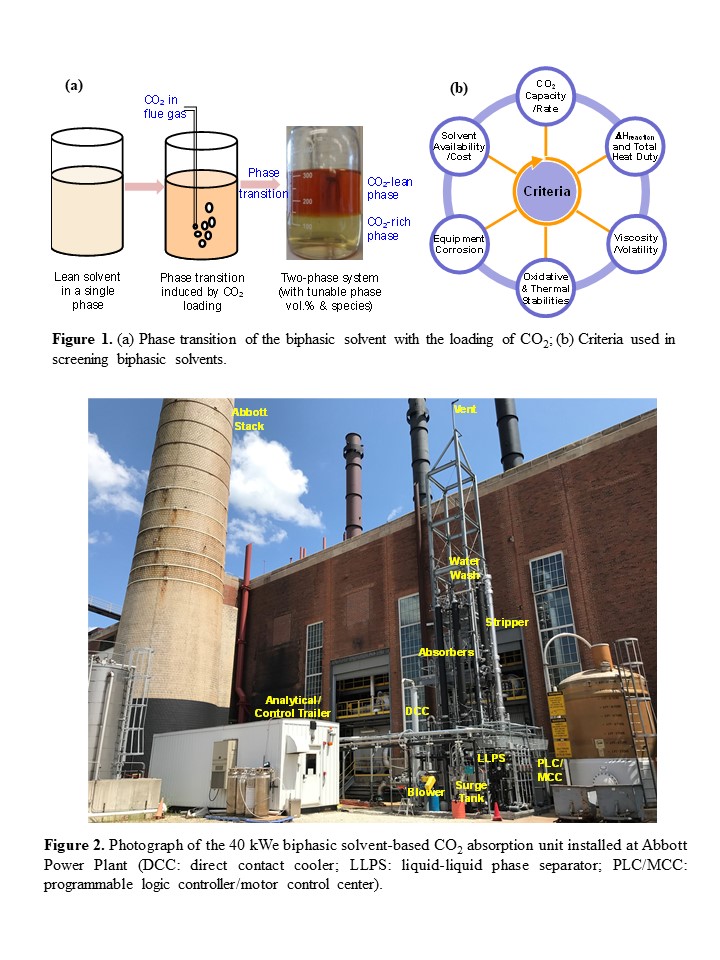
In this study, a new group of biphasic solvents have been developed. These solvents are a blend of chemicals that enhance both capacity and rate in solutions with low water content. The solvent exists as a single phase at low to no CO2 loading, but it undergoes a phase transition to two liquid phases upon CO2 loading (see Figure 1a). In the laboratory, approximately 80 biphasic solvents were screened based on multiple criteria, including working capacity, absorption rate, thermal and oxidative stabilities, viscosity, and corrosion tendency (see Figure 1b). Two biphasic solvents emerged as superior to all others. Notably, these two biphasic solvents exhibited high resistance to both oxygen-containing and thermal environments. Thermal incubation measurements revealed that the thermal stability of each biphasic solvent for 2 weeks at temperature as high as 150 °C was similar to that of the benchmark 30 wt.% monoethanolamide (MEA) solvent at 120 °C. Oxidative stability measurements showed that compared to MEA, the biphasic solvents exhibited ~8 times higher resistance to oxygen when bubbled in a gas mixture of 96% O2 and 4% CO2 at 50 °C for a period of 2 weeks. Furthermore, under simulated absorption and desorption conditions, the corrosion rates of the two biphasic solvents, as measured with a carbon steel coupon (CS1010), were 2-3 times lower than that of MEA.
To evaluate the performance of the developed biphasic solvents, a 40 kWe bench-scale capture skid was built and installed at the University of Illinois’ Abbott power plant (see Figure 2). The skid is an integrated system comprising the flue gas polishing, CO2 absorption, phase separation, and desorption steps. Parametric tests were conducted for the two biphasic solvents and the 30 wt.% MEA solution, with respect to multiple process or operating variables such as liquid-to-gas ratio, CO2 concentration, stripping pressure/temperature, and CO2 loading, to validate and compare the performance and operational flexibility of the skid. The results of the parametric tests showed that both biphasic solvents appeared to be more energy efficient than the reference MEA. Under representative operating conditions, the regeneration heat duty required for the two biphasic solvents was reduced by ~40-43%, and the stripping pressures was elevated by ~3 times compared with MEA. After completing the parametric testing, continuous 24/7 testing was performed with a slipstream of actual coal combustion flue gas from the power plant for one selected biphasic solvent over a course of totally four weeks in two test campaigns. Both test campaigns were operated under steady state at pre-selected conditions. The first campaign aimed for a 90% CO2 removal rate, while the second one targeted a 95% removal rate with the stripping pressure slightly adjusted. During the slipstream testing, continuous, steady-state operations were validated during the cold wintertime. Test results also revealed that the heat duty required for solvent regeneration ranged between 2,180 and 2,450 MJ/tonne of CO2 captured, and the stripping pressure ranged between 65 and 60 psia, depending on the CO2 removal rate target (i.e., 90% or 95%).
We further conducted a techno-economic analysis (TEA) study to evaluate the biphasic solvent-enabled absorption process for large-scale PCC applications. The TEA study followed the methodology and assumptions consistent with those used in the US Department of Energy (USDOE)’s cost baseline study (Version of Revision 4). The results of the TEA study revealed that the parasitic power loss for the biphasic solvent-based process installed in a net 650 MWe power plant could be reduced by ~19% and the cost of CO2 capture by ~20% compared with the State-of-the-Art technology adopted in USDOE’s Base Case B12B. Such technoeconomic advantages resulted from the higher energy efficiency for both CO2 capture and power generation, smaller power plant for the same net output, and lower capital costs associated with CO2 absorption, stripping, and compression equipment. This work will continue to investigate and test the performance of the biphasic solvent-based process for CO2 capture from other industrial point sources such as waste-to-energy facilities.