(678c) Monitoring the Electrocatalytic Activity and Material Stability of Cobalt in Acidic Media during Oxygen and Hydrogen Electrocatalysis
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Fundamentals of Catalysis and Surface Science IV: Electrocatalysis
Thursday, November 9, 2023 - 1:06pm to 1:24pm
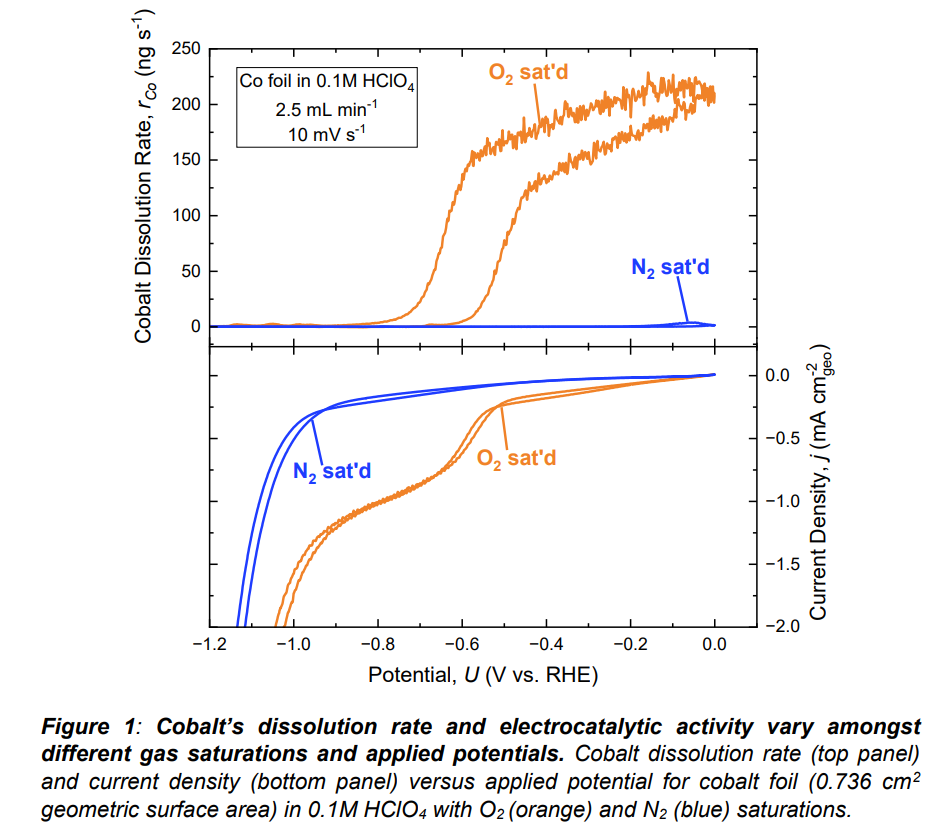
Cobalt (Co) thermodynamically has a large potential window of instability in acidic media that negatively impacts its usage in energy devices. In particular, leaching of Co from the PtCo/C catalyst cathode in commercial proton-exchange membrane fuel cells (PEMFCs) limits the fuel cell’s overall performance and lifetime. Minimizing electrode degradation while ensuring optimal performance of Co-based materials requires a more fundamental understanding of cobalt’s material stability in acidic media. In this work, we simultaneously monitor the electrocatalytic activity and material stability of Co foil during the oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER) in 0.1M HClO4 by coupling ICP-MS (inductively coupled plasma mass spectrometry) with an electrochemical flow cell (known as on-line ICP-MS). To evaluate the role of catalysis on cobalt’s material stability, we compare Co dissolution in O2-, N2-, and H2-saturated electrolyte in which we observe a ~140-fold increase in dissolved Co under O2 saturation relative to N2/H2 saturation at 0 VRHE despite negligible faradaic current. These results indicate that the presence of O2 destabilizes the Co surface in the absence of electrocatalysis. However, at potentials below cobalt’s ORR onset potential (-0.55 VRHE @ -0.2 mA cm-2), Co stabilizes and there is < 3.5 ng s-1 dissolution at -0.8 VRHE under all gas saturations despite the magnitude of current density differing by ~0.8 mA cm-2 between O2 and N2/H2 saturation. These results suggest that electrocatalysis stabilizes the Co surface. To further investigate the stabilization mechanisms of Co, we employ electrochemical mass spectrometry (EC-MS) to measure the faradaic efficiency towards HER. Altogether, this work provides important insights to better understand the degradation mechanism of Co in acidic media under reaction conditions. Such understanding will motivate future studies to improve the stability of Co-based materials for a variety of applications, including fuel cells, electrolyzers, and lithium-ion batteries.