(677f) Elucidating the Influence of Water-Saturated Cs on Phenol Tautomerization
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Fundamentals of Catalysis and Surface Science III: Catalysis over Metals
Thursday, November 9, 2023 - 9:30am to 9:48am
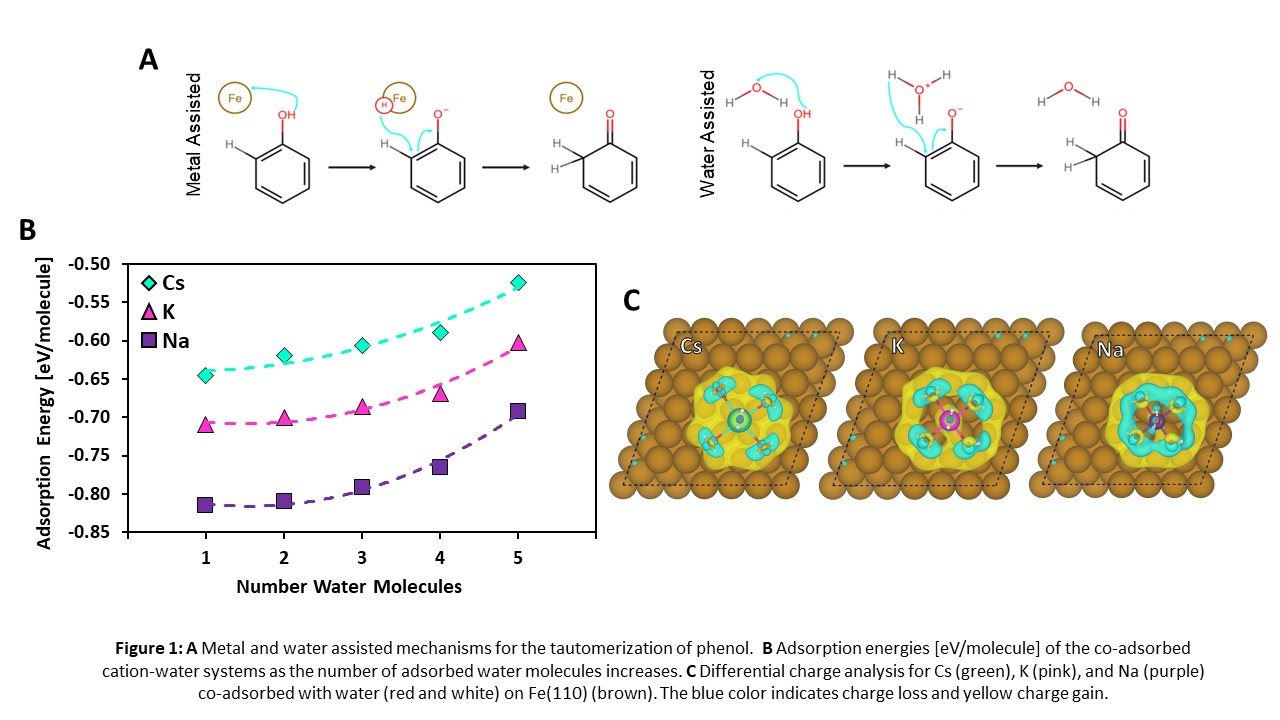
DFT results found that water molecules preferentially bind to Cs compared to Fe, suggesting a likelihood of water molecules forming around the Cs atom. The adsorption energy of water per molecule decreases as more water molecules are adsorbed up to the saturation point. This trend is consistent regardless of the cation (Fig 1B), where it was further determined that the co-adsorption of alkalis and water decreases as the cation size increases (Na>K>Cs). The differential charge analysis (Fig 1C) indicates an increasing amount of charge transfer from the cation to Fe (Na<K<Cs), consistent with the larger Na-system adsorption energies. This stronger adsorption is attributed to the smaller size of the Na cation, leading to shorter water-Na bond distances and constricting of the cation-water network, which limits the charge transfer from Na to Fe.
The reaction pathways are illuminated via the Nudge Elastic Band (NEB) method, where the adsorption energies of the initial, intermediary, and final steps indicate a high energy barrier to remove the hydrogen from the -OH group of phenol. Results from this study will aid in the understanding of the dual impact of local electric fields and solvent environments on a vital HDO mechanism to upgrade biofuels.