(676b) Contrasting Methylating Agents (CH3XHn, X={N, P, O, S, F, Cl}) for Solid-Acid Catalyzed Reactions to Re-Examine Acid Strength and Confinement Effects
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Fundamentals of Catalysis and Surface Science II: C1 Catalysis
Wednesday, November 8, 2023 - 12:48pm to 1:06pm
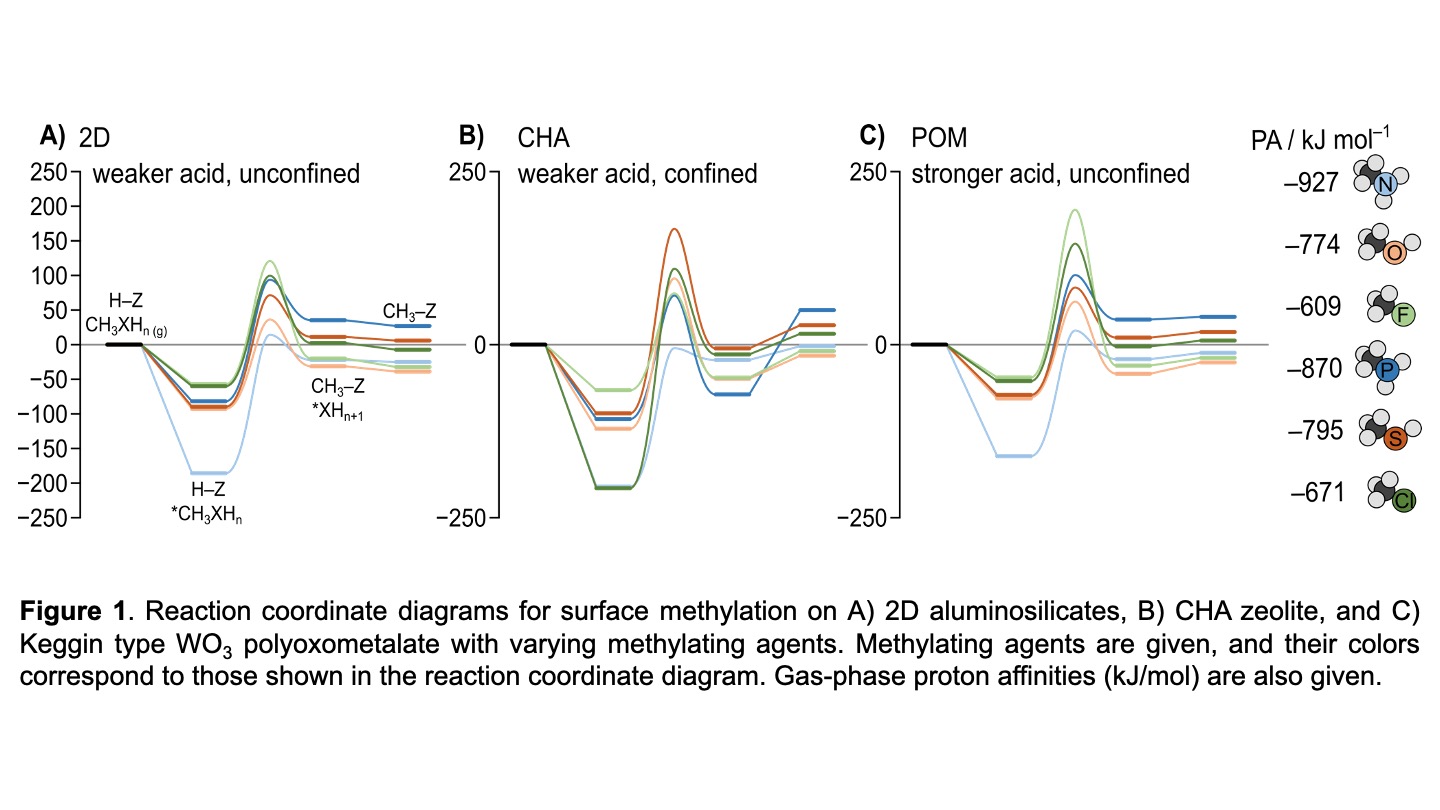
Methanol dehydration to dimethyl ether (DME) is a probe reaction previously used to access and deconvolute acid strength and confinement effects for various solid Brønsted acid catalysts. Among them are zeolites with varying confinement and topology, and polyoxometalates with varying acid strength. Methanol dehydration can occur via two competing mechanisms, a concerted (associative) or sequential (dissociative), where the former forms DME in a single bimolecular reaction, while the latter forms DME via a surface-bound methyl (Z–CH3) intermediate. Density functional theory (DFT) calculations suggest that methanol dehydrates via the concerted route at typical reaction conditions (< 450 K, >0.3 kPa CH3OH). Here, we perform DFT calculations to revisit the methanol dehydration reaction by systematically varying functional (electronic) groups from –OH in methanol to –NH2, –PH2, –SH, –F, and –Cl in methylamine, methyl phosphine, methanethiol, methyl fluoride, and methyl chloride, respectively, to fundamentally address if the conclusions from methanol dehydration about Brønsted acid catalysts are extendible to other probe molecules. Specifically, we revisit the role of zeolite topology and confinement effects by contrasting CHA and MFI zeolite frameworks to a 2D surface model of a zeolite, and we revisit the role of acid strength in Keggin type WO3 polyoxometalates (POMs) by varying the metal heteroatom identity (Al, Si, P, and S). While we have examined all three steps involved in methanol dehydration (surface methylation, methanol methylation, and concerted DME formation), here we present exemplar data on surface methylation by the CH3XHn series that indicate the most facile surface methylations occur with CH3NH2 donors, followed by CH3OH, CH3SH, CH3PH2, CH3Cl, and the weakest methyl donor being CH3F. While proton affinities describe differences between functional groups, reaction energies suggest that confinement and acid strength alters probe molecule behavior to different extents. This, in turn, motivates revisiting further conclusions derived from probe reaction chemistry.