(659b) Development and Application of Detailed Kinetics Mechanisms for PFAS Incineration
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Combustion Kinetics and Emissions
Sunday, November 5, 2023 - 3:50pm to 4:10pm
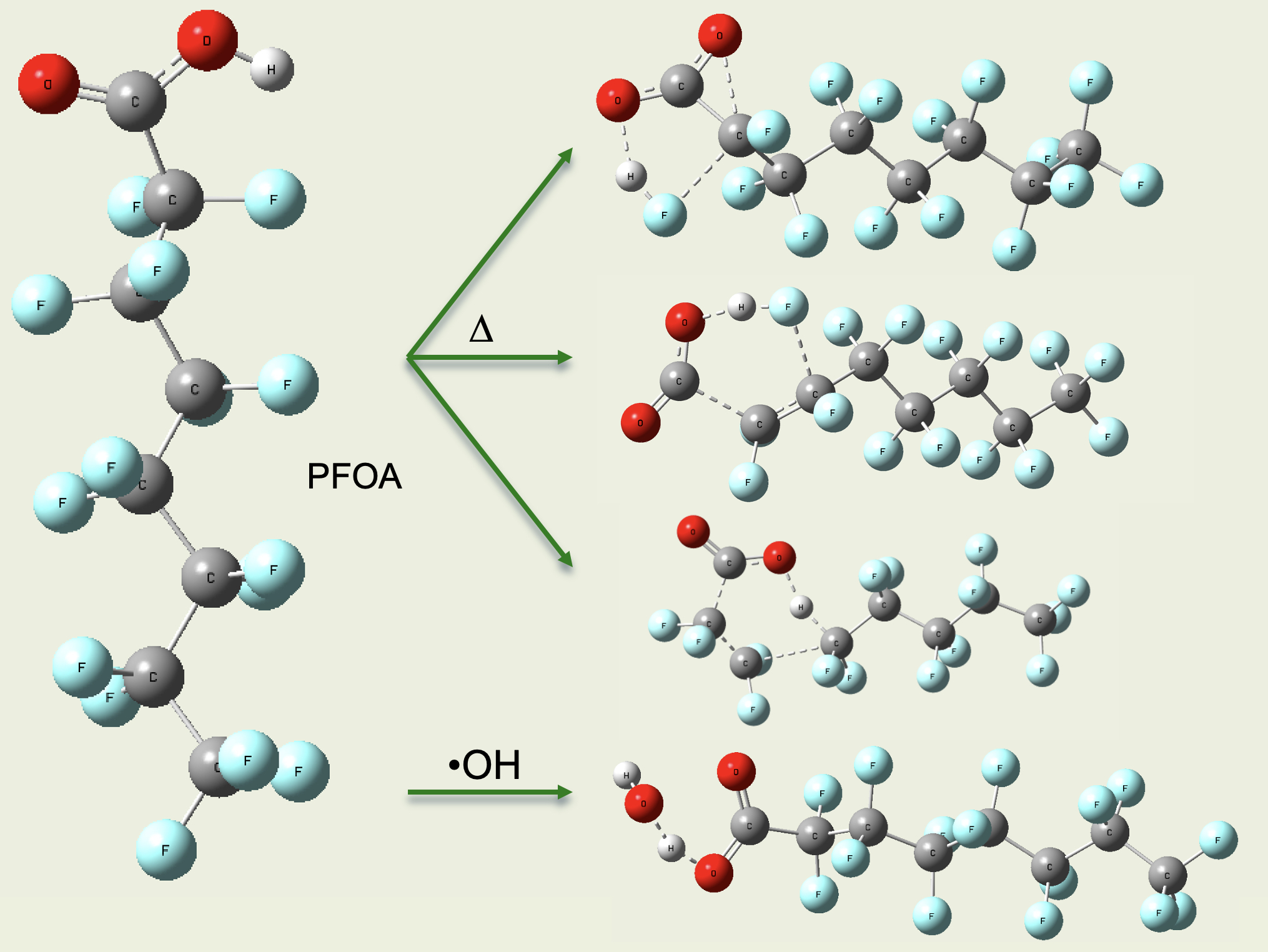
Recent data provide the basis for testing detailed kinetic mechanisms that we have predicted for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS). The necessary chemical intermediates, thermochemistry, reactions, rate expressions, and transport properties have been developed using computational quantum chemistry (Gaussian at B3LYP/6-31(G(d,p) and M06-2x/6-311+G(d,p) levels), ideal-gas statistical mechanics and reaction theory (Arkane), and correlations. Testing for pyrolysis, flame, and post-flame reactor performance has primarily used the reactive-flow models of ANSYS Chemkin Pro.
Modeling predictions show that pericyclic decompositions dominate pyrolysis, not radical chemistry, mainly because of the strong C-F bonds and lack of abstractable Hs. The lowest-energy decompositions from PFOA are HF elimination to make a perfluoroalkane that ends in a "lactone" three-membered -(CF-CO-O-) ring, decomposing to CO or CO2; a perfluoro alpha-olefin; or a 1H-perfluoroalkane. In a flame, radicals can abstract the lone H in PFOA or PFOS, respectively generating a perfluorocarboxylate or sulfonate that decomposes to release CO2 or SO3, although the PFAS decompositions still compete. In conventional post-flame incineration, PFAS decompositions appear to dominate.
Predictions will be tested and refined by comparison to experiments in the EPA Rainbow pilot-plant incinerator, recognizing that practical incinerators do not feed PFOA or PFOS per se but instead ionic solutions of these compounds. These findings point to control strategies for making incineration effective for the PFAS feed and for combustion intermediates.