(626e) Interfacial Instability Due to Electrodeposition: Analysis of Galvanostatic Case
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Engineering Sciences and Fundamentals
Solid-Liquid Interfaces
Sunday, November 5, 2023 - 4:30pm to 4:45pm
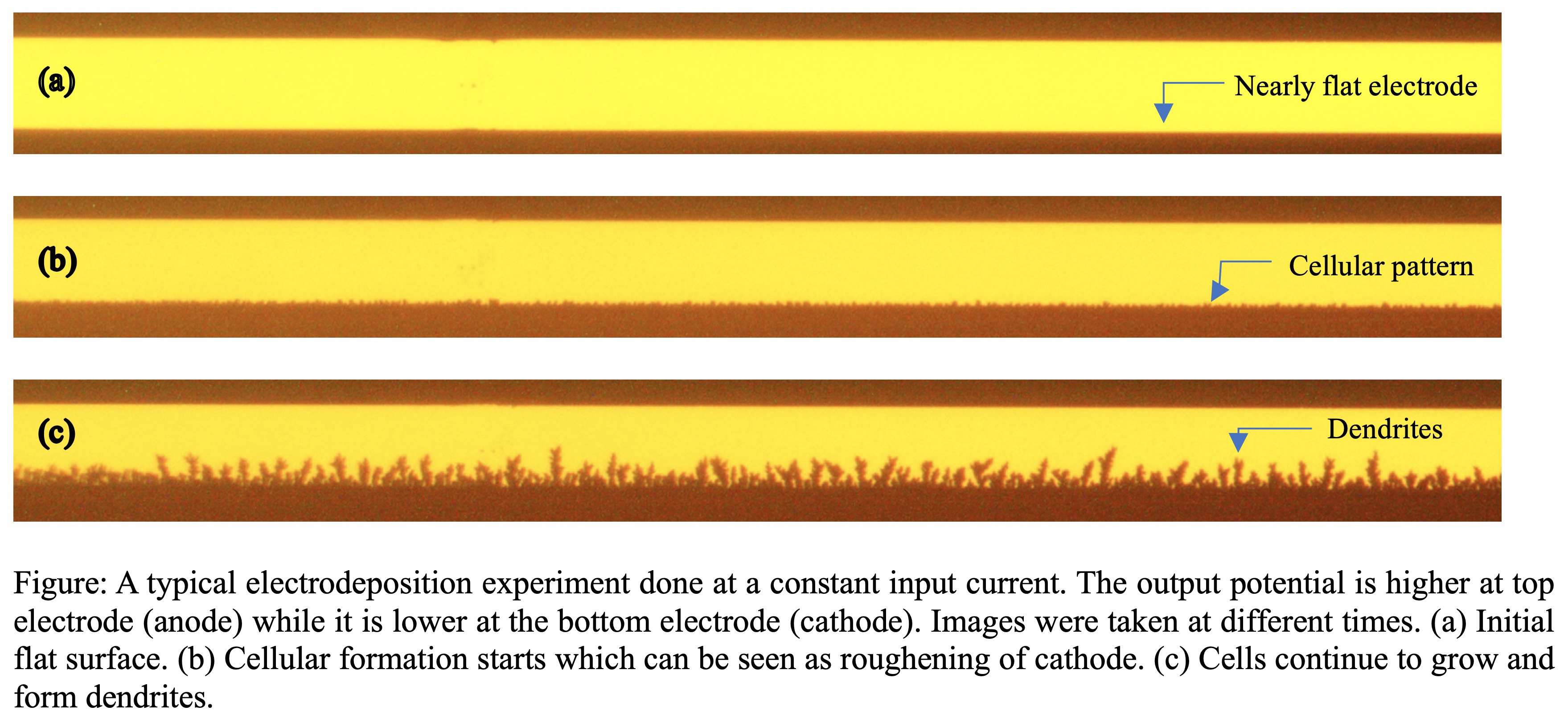
In this work, we propose a general model that predicts the onset and growth of interfacial patterns at a cathodic surface when electric current is supplied to an electrochemical cell, which is composed of two metal electrodes separated by a dilute aqueous metal-ion solution. The patterns at a cathode surface result from an instability during electrodeposition that takes the form of organized interfacial waves despite random perturbations or disturbances. These wave-like cellular patterns become dendritic in form as time progresses. For this instability, the growth rate of patterns reaches a maximum value at a particular wavenumber which depends on the scaled input current, the scaled mean concentration of the aqueous metal solution, and the scaled surface energy. These models are compared to earlier simple models where local electroneutrality is assumed, a drastic assumption that becomes questionable specifically near the electrodes where the instability is taking place. Moreover, domain dynamics are also ignored in the earlier model (BuAli et. al., 2006). The objective is to inquire when the predictions done by earlier model might fail to coincide with the experimental observations. The predictions made by this model are also compared with experiments done for copper electrodes that span a gap containing dilute copper sulfate solution. This instability resembles many other interfacial instabilities like Rayleigh-Taylor instability and Marangoni instability, where the growth rate is principally controlled by interfacial dynamics.