(579e) Metabolic Modeling of Eicosanoid Class Switch during Arachidonic Acid Metabolism in Mouse Macrophage Cells
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Food, Pharmaceutical & Bioengineering Division
Metabolic Engineering - Applications in Health
Tuesday, November 7, 2023 - 1:42pm to 2:00pm
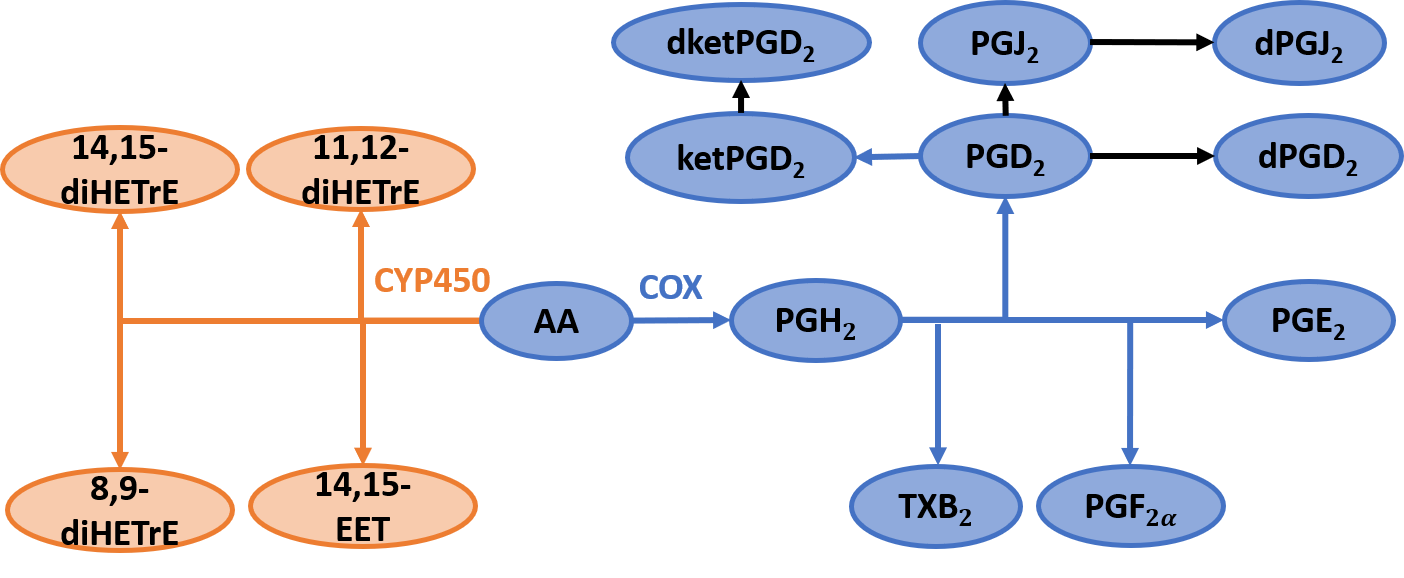
This study aims to mathematically model the eicosanoid class switch in AA metabolism during the inflammatory response. The cybernetic framework, a metabolic modeling technique, is utilized to model the reaction network (shown below). The cyclooxygenase (COX) and cytochromeP450 (CP450) pathways are included in the model. Prostaglandins, formed due to COX activation, are pro-inflammatory; whereas EET and dihydroxyeicosatrienoic (diHETrE), CP450 products, are anti-inflammatory. The cybernetic framework assumes a biological goal. The cybernetic goal is defined and correspondingly the values of cybernetic control variables are computed. The biological goal is proposed as maximizing the pro- and anti-inflammatory responses. The cybernetic goal is formulated as maximizing the sum of the production rates of COX (pro-inflammatory) and CYP450 (anti-inflammatory) products. The cybernetic goal and control variable formulations are shown below. The parameters will be estimated and the model will be simulated to capture the experimental data. The control variables will display eicosanoid class switching, where the pro-inflammatory phase will dominate for a duration, and then the anti-inflammatory response will take over.
Cybernetic goal:
max [ÏPGH2 +Ï14,15-EET+ Ï14,15-diHETrE +Ï8,9-diHETrE + Ï11,12-diHETrE]
Where Ï represents the reaction flux of the metabolite.
Cybernetic control variables:
uPGH2 =ÏPGH2 / (â¡ÏPGH2 +Ï14,15-EET+ Ï14,15-diHETrE +Ï8,9-diHETrE + Ï11,12-diHETrE )
u14,15-EET =Ï14,15-EET/(â¡ÏPGH2 +Ï14,15-EET+ Ï14,15-diHETrE +Ï8,9-diHETrE + Ï11,12-diHETrE)
u14,15-diHETrE =Ï14,15-diHETrE/(â¡ÏPGH2 +Ï14,15-EET+ Ï14,15-diHETrE +Ï8,9-diHETrE + Ï11,12-diHETrE)
u8,9-diHETrE =Ï8,9-diHETrE/(â¡ÏPGH2 +Ï14,15-EET+ Ï14,15-diHETrE +Ï8,9-diHETrE + Ï11,12-diHETrE )
u11,12-diHETrE =1 - [uPGH2 + u14,15-EET+ u14,15-diHETrE+u_(8,9-diHETrE) ]
vPGH2 =ÏPGH2/(maxâ¡[ÏPGH2 , Ï14,15-EET , Ï14,15-diHETrE , Ï8,9-diHETrE , Ï11,12-diHETrE )
v14,15-EET =Ï14,15-EET/(maxâ¡[ÏPGH2 , Ï14,15-EET , Ï14,15-diHETrE , Ï8,9-diHETrE , Ï11,12-diHETrE )
v14,15-diHETrE =Ï14,15-diHETrE/(maxâ¡[ÏPGH2 , Ï14,15-EET , Ï14,15-diHETrE , Ï8,9-diHETrE , Ï11,12-diHETrE )
v8,9-diHETrE =Ï8,9-diHETrE/(maxâ¡[ÏPGH2 , Ï14,15-EET , Ï14,15-diHETrE , Ï8,9-diHETrE , Ï11,12-diHETrE )
v11,12-diHETrE =Ï11,12-diHETrE/(maxâ¡[ÏPGH2 , Ï14,15-EET , Ï14,15-diHETrE , Ï8,9-diHETrE , Ï11,12-diHETrE )