(539c) Investigating Structure-Performance Relationships in Ligand-Functionalized Membranes for Lithium Ion-Selective Separations
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Separations Division
Membranes Designed for Ion-Ion Separations II
Wednesday, November 8, 2023 - 4:12pm to 4:33pm
Selective electrodialysis requires further development of ion-selective membranes to enable ion-selective recovery from aqueous streams. In this work, we evaluated a library of commercial membranes to assess their potential for ion-ion separations and to elucidate the fundamental mechanisms of ion selectivity in these materials. This screening of commercial membranes confirmed the limitations of size and electrostatic selectivity mechanisms in the separation of ions of similar size and like charge. Ongoing work focuses on the synthesis and testing of novel ligand-functionalized membranes designed for enhanced ion selectivity via ion-specific coordinative interactions.
Introduction
With global lithium supplies projected to fall short of demand between 2023 and 2027, there is great motivation to develop new technologies capable of lithium recovery from alternative sources such as battery waste, produced water and geothermal brines (Kumar, 2019). In these sources, the co-existence of other impurities at much higher concentrations than lithium (e.g., >60,000 ppm Na+ versus 50-1000 ppm Li+ in brines) presents a challenge for high-purity lithium extraction (Daitch, 2018; Leece, 2022). Selective electrodialysis is a candidate for lithium recovery that aligns with recent resource extraction priorities including developing electrified separation processes; however, it requires the development of ion-selective membranes (Cath, 2021). To inform the design of such membranes, we first studied fundamental ion transport and selectivity mechanisms in commercially available membranes. We then synthesized a library of novel ligand-functionalized membranes to study the impact of additional ion-specific coordinative interactions on lithium selectivity during selective electrodialysis.
Methods
Membrane Performance Testing
Diffusion experiments were performed with single-salt and binary electrolyte feed solutions (0.1 M LiCl, 0.1 M competing NaCl, MgCl2, NiCl2, or CoCl2) and nanopure water receiving solutions in custom H-cells 3D-printed from an epoxy polyacrylate photopolymer resin. A membrane with 2.01 cm2 cross-sectional area was mounted between the 40 mL feed and receiving chambers. Feed and receiving chamber compositions were monitored over a period of eight hours via ion chromatography to calculate cation flux, permeability, and selectivity.
Novel Ligand-Functionalized Membrane Synthesis and Characterization
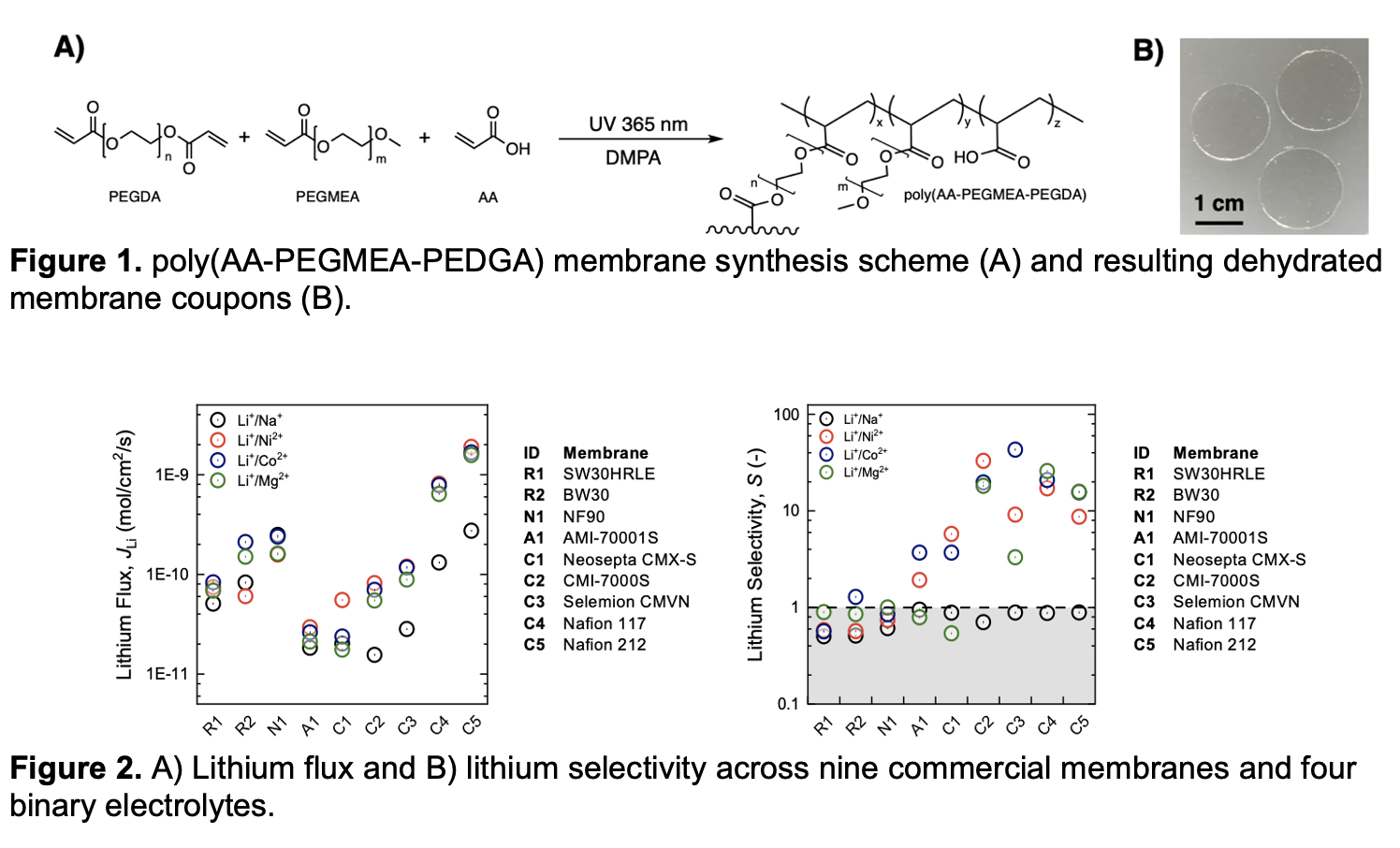
Several 0.5 mm thick membranes were fabricated via free-radical UV photopolymerization with poly(ethylene glycol) methyl ether acrylate (PEGMEA) and poly(ethylene glycol) diacrylate (PEGDA) using 2,2-dimethoxy-2-phenylacetophenone (DMPA) as the photo-initiator. Co-monomers containing desired functional groups (e.g., carboxylic acid, pyridine moieties) were incorporated into membranes (Figure 1). Membrane characterization includes composition confirmation via gel fraction, FTIR, and elemental analysis, as well as water uptake.
Results and Discussion
Screening ion permeability and selectivity in commercial membranes
Nine commercial membranes, ranging from reverse osmosis to ion-exchange membranes, were evaluated in diffusive ion permeation studies for four binary electrolytes relevant to lithium-rich brines and battery leachates (LiCl/NaCl, LiCl/MgCl2, LiCl/NiCl2, LiCl/CoCl2). Lithium flux and selectivity results are presented in Figure 2. This experimental screening represents a significant expansion of lithium permeability and selectivity data reported in literature, with the most recent compilation of experimental results containing 12 data points for LiCl/NaCl systems only (Sujanani, 2020). Cation exchange membranes demonstrate some diffusive selectivity for monovalent over divalent ions thanks to the valence-dependency of counterion diffusivity in ion exchange membranes, as seen in the experimental results (Fan, 2022); however, selectivity between monovalent ions (e.g., Li and Na) remains elusive as monovalent ions are indistinguishable in valence and are very similar in size. As a result, size and electrostatic selectivity mechanisms in ion exchange membranes are ineffective for separation of ions of the same valence.
Fabrication and Characterization of Novel Ligand-Functionalized Membranes
The introduction of additional ion-specific coordinative interactions can help achieve selectivity between ions with the same valence (e.g., monovalent cations). In membranes containing ligands that display different ion-coordination behaviors with the ions of separation interest, the varying strengths of such ion-ligand coordination behaviors (which can be described by ion-ligand interaction free energies) introduces an additional selectivity mechanism beyond size and valence selectivity mechanisms in traditional ion exchange membranes. This potential motivates our current work of synthesizing a library of membranes grafted with select ligands at varying density for a systematic study of their effects on ion transport. While previous simulation work has studied the effect of ligand-cation interaction free energy on ion permeabilities (Zofchak, 2022), the additional effect of ligand density within the membrane polymeric network has not specifically been investigated to our knowledge. As such, our testing of extensive libraries of ligand-functionalized membranes with variable ligand species and grafting densities (and other variables such as membrane water uptake held constant) will provide a clearer picture of the structure-function relationships of membranes pertinent to ion-selective separation applications. Preliminary data show variation in permeability and selectivity with ligand grafting density, suggesting that this is an additional design variable requiring consideration in the design of membranes for ion-ion separations. These results will help elucidate fundamental barriers that limit ion permselectivity in the context of lithium recovery.
Conclusions and Implications
This work includes the study of fundamental ion transport and selectivity mechanisms in both commercially available membrane and novel ligand-functionalized membranes, with the model application of lithium recovery via selective electrodialysis. Regarding the screening of commercial membranes, our standardized comparison of ion transport in commercial membranes advances toward more comprehensive datasets for lithium-selective separations. Our 36 experimental datapoints for LiCl/{NaCl, MgCl2, NiCl2, CoCl2} separations is a significant expansion of the 12 aggregated datapoints reported previously (Sujanani, 2020). This performance comparison across existing membrane technologies provided critical insights on the limitations of these non-porous and ion exchange membranes, motivating the development of novel membranes with additional selectivity mechanisms to enhance performance. The complete characterization of the library of novel, ligand-functionalized membranes provides additional experimental data on the effect of not only ligand-cation interaction free energies, but also the effect of ligand grafting densities on ion permeabilities and selectivities. In the future, these experimental results could support further development of ion transport theory in ligand-functionalized membranes with ligand grafting densities included as model parameters. This work could also lead to future grafting density optimization studies with the goal of maximizing ion permeability and/or selectivity.
References
Adam Leece, Dreis, M., Bartholameuz, E., Vypovska, A., & Majeti, M. (2022). Economic Assessment of Lithium Production Potential from Canadian Oil and Gas Operations. Canada Energy Research Institute. https://ceri.ca/assets/files/Study_198_Full_Report.pdf
Cath, T., Chellam, S., Katz, L., Breckenridge, R., Ellison, K., Macknick, J., Monnell, J., Rao, N., Sedlak, D., & Stokes-Draut, J. (2021). National Alliance for Water Innovation (NAWI) Technology Roadmap: Resource Extraction Sector. DOE/ GO-102021-5567. 130.
Fan, H., Huang, Y., Billinge, I. H., Bannon, S. M., Geise, G. M., & Yip, N. Y. (2022). Counterion Mobility in Ion-Exchange Membranes: Spatial Effect and Valency-Dependent Electrostatic Interaction. ACS ES&T Engineering, acsestengg.1c00457. https://doi.org/10.1021/acsestengg.1c00457
Kumar, A., Fukuda, H., Hatton, T. A., & Lienhard, J. H. (2019). Lithium Recovery from Oil and Gas Produced Water: A Need for a Growing Energy Industry. ACS Energy Letters, 4(6), 1471–1474. https://doi.org/10.1021/acsenergylett.9b00779
Pamela Joy Daitch. (2018). Lithium extraction from oilfield brine [University of Texas at Austin]. https://repositories.lib.utexas.edu/bitstream/handle/2152/65645/DAITCH-T...
Sujanani, R., Landsman, M. R., Jiao, S., Moon, J. D., Shell, M. S., Lawler, D. F., Katz, L. E., & Freeman, B. D. (2020). Designing Solute-Tailored Selectivity in Membranes: Perspectives for Water Reuse and Resource Recovery. ACS Macro Letters, 9(11), 1709–1717. https://doi.org/10.1021/acsmacrolett.0c00710
Zofchak, E. S., Zhang, Z., Marioni, N., Duncan, T. J., Sachar, H. S., Chamseddine, A., Freeman, B. D., & Ganesan, V. (2022). Cation–Ligand Interactions Dictate Salt Partitioning and Diffusivity in Ligand-Functionalized Polymer Membranes. Macromolecules, 55(6), 2260–2270. https://doi.org/10.1021/acs.macromol.2c00035