(521o) Understanding the Adsorptive Behavior of Polymeric Materials to Catalyst Surfaces Using Various X-Ray Methods
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Poster Session: Catalysis and Reaction Engineering (CRE) Division
Wednesday, November 8, 2023 - 3:30pm to 5:00pm
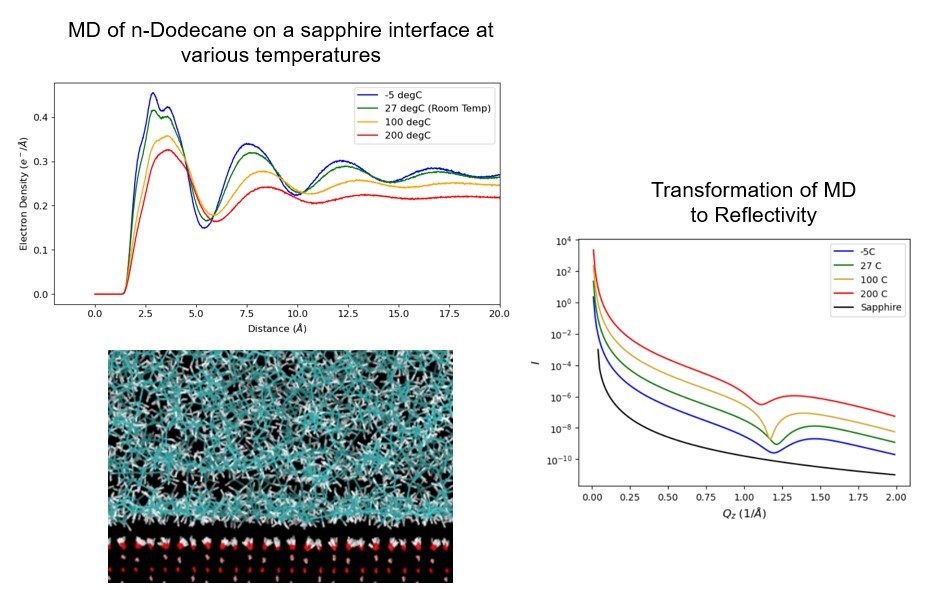
To study the arrangement of hydrocarbons to the catalyst support, we utilize X-Ray Reflectivity (XRR) and Molecular Dynamics (MD) simulations, and to study the local chemical environment changes of the Ru active site, we utilize Extended X-Ray Adsorption Fine Edge Structure (EXAFS). By utilizing hydrocarbons of various lengths (methane, hexane, dodecane, triacontane, and low-density polyethylene), we will understand preferential adsorption arrangements for undesirable and desirable product species.
We theorize that as hydrocarbon chain length increases, the polymer will adsorb to the support with increased structural order, resulting in increased adsorption potential to the Ru active site. MD simulations of n-dodecane at various temperatures indicates that the preferential adsorption of species parallel to an alumina interface changes, and the application of the distorted crystal model confirms the viability of XRR as an experimental technique, as seen in the attached image. EXAFS data is currently being processed and will yield information about the local relaxation of Ru nanoparticles during species adsorption.
This work aims to understand the structure and arrangement of hydrocarbons to the active metal site and the support, thus allowing for fundamental concepts to be unified and expanded upon. We anticipate our results to inspire future surface science studies to explore how different polymers adsorb to the catalyst surface—ultimately allowing for the creation of catalysts capable of mixed-polymer hydrogenolysis and reduction of energy requirements for polymer upcycling.