(521bh) Manipulating Inner and Outer Sphere Environments in Zeolites to Control Regioselectivity of Epoxide Methanolysis
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Poster Session: Catalysis and Reaction Engineering (CRE) Division
Wednesday, November 8, 2023 - 3:30pm to 5:00pm
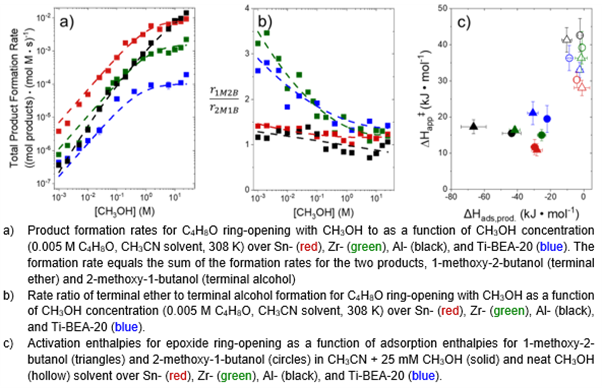
Among Lewis acids, ring-opening rates proceed in the order Ti < Zr < Sn, correlating with reported metal Lewis acid strengths. Rates depend on [CH3OH] (where [x] denotes concentration) and [C4H8O]. A single kinetic regime appears to dominate at all conditions over Al-BEA-20, whereas each Lewis acid shows two kinetic regimes. These results suggest that Lewis and Brønsted acidic materials catalyze ring-opening with distinct prevailing surface species or through different mechanisms.
Regioselectivities towards the terminal ether product exceed 50% under most conditions but depend on both metal identity and reagent concentrations. Selectivity towards the terminal ether generally decreases with increasing [CH3OH] and increases with greater [C4H8O] over each M-BEA-20. Al-BEA-20 consistently gives the lowest regioselectivities to the terminal ether, with Ti- and Zr-BEA-20 showing the greatest regioselectivities. Activation enthalpy and reactant and product adsorption enthalpies, obtained by isothermal titration calorimetry, suggest regioselectivities stem from differences in the free energies of the transition states to form each product. Methanol uptake measurements further suggest that differences in solvation between transition states contribute to regioselectivity changes.
This work demonstrates that regioselectivities for epoxide ring-opening are controlled by both inner sphere (i.e., choice of active metal, acid type) and outer sphere (i.e., intrapore solvent environment) interactions in zeolite pores.