(502c) Nuclear Egress of Cytokinetic Proteins Promotes Migration, Invasion and Metastasis
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Food, Pharmaceutical & Bioengineering Division
Decoding the Cell Microenvironment: Chemical and Physical Signaling and Cellular Responses
Wednesday, November 8, 2023 - 8:36am to 8:54am
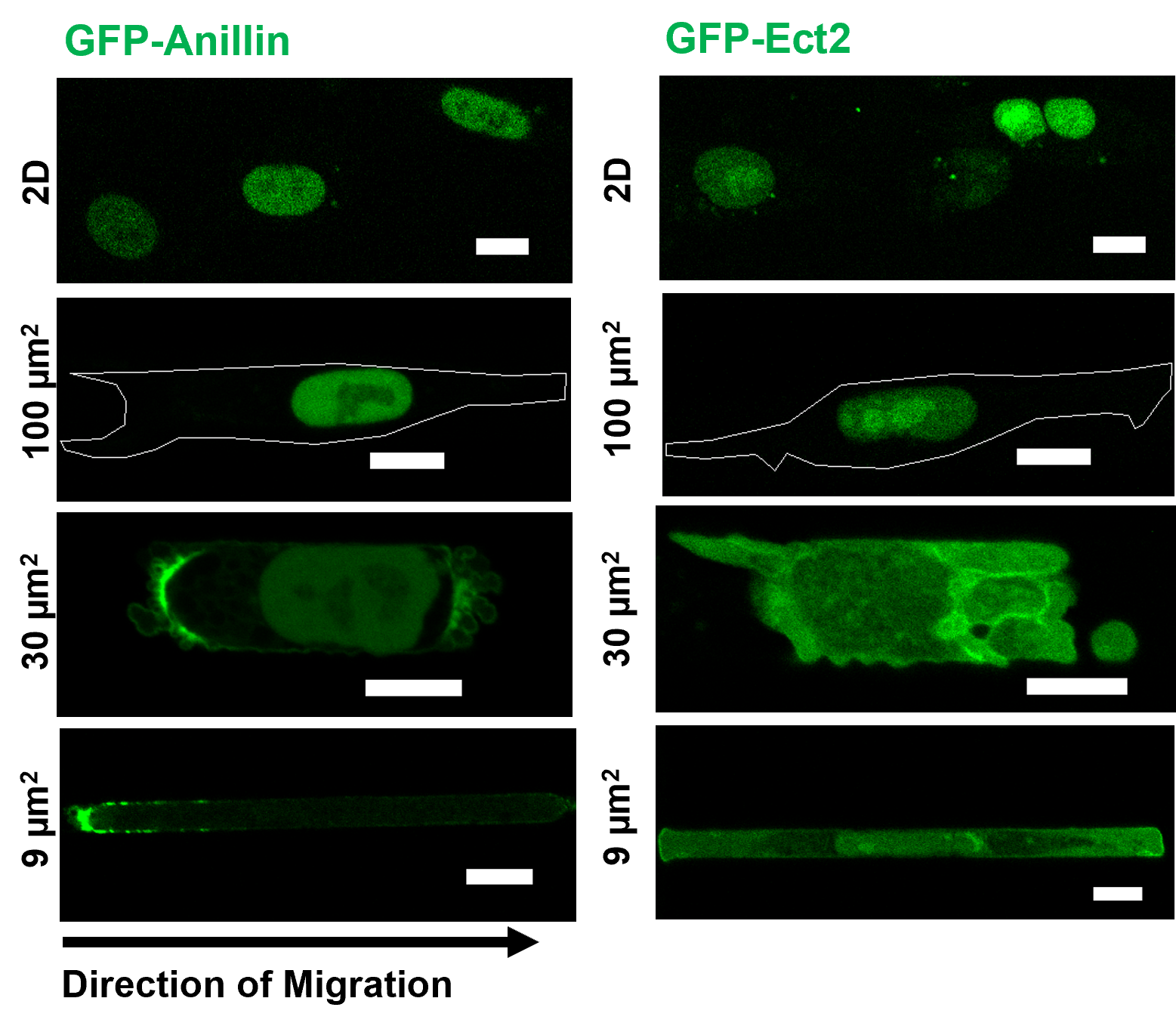
Metastasis is the leading cause of death for cancer patients. During metastasis, cells have to navigate through confining spaces and experience mechanical challenges that results in transient nuclear envelope ruptures, which can have deleterious effects on the cell genome. Many questions remain regarding how nuclear ruptures may affect cell migration and its implication for cancer cell metastasis. By utilizing a combination of both stiff and compliant microfluidic devices and animal models, we observed how nuclear confinement leads to nuclear envelope rupture and subsequently cytoplasmic translocation of the scaffolding protein anillin and the RhoGEF Ect2, both of which are cytokinetic proteins predominantly residing in the nucleus under normal conditions. Anillin, in particular, accumulates at the cell poles and the degree of accumulation scales with the extent of microenvironmental stiffness and confinement. Accumulation is lower on softer 8 kPa substrate compared to 21 kPa, but can be enhanced by tighter confinement with narrower channels. This phenomenon can be observed in cells migrating in microfluidic devices, 3D collagen gel, invading tracks between myofibers in mouse deep dermis, and in chick embryo cholioallantoic membrane (CAM), typically after a nuclear rupture event. This coincides with increased RhoA polarization and activity in confinement. Inhibiting RhoA via pharmalogical means greatly impede cell entry into confined microchannels, in both the time required for cell entry and the proportion of cells able to enter. To probe the relationship between anillin and Ect2 cytoplasmic accumulation, cell contractility and cell migration, we expressed various functional mutants of anillin and Ect2 in HT1080 and studied their effects both in vitro and in vivo. Simulating anillin and Ect2 nuclear exit by simultaneous overexpression of constructs without the nuclear localization signals in unconfined cells significantly increase phosphorylated-MLC level, indicating enhanced contractility. In return, expression of an anillin truncated mutant incapable of binding actin and myosin together with an Ect2 variant with mutations in the GEF catalytic domain decrease overall contractility. This results in impaired cell entry into confined microchannels, reduced cell dissociation from spheroids encapsulated in collagen gel, and fewer cells invading from the main tumor mass into the surrounding tissue in chick embryo CAM. Taken together, we propose that tight nuclear confinement leads to transient nuclear envelope rupture, releasing anillin and Ect2 from the nucleus into the cytoplasm. Through the former’s binding to actin and myosin and the latter’s RhoGEF activity, cell contractility is elevated, promoting efficient migration and invasion. Overall, our work identifies nuclear envelope rupture and subsequent translocation of cytokinetic proteins as a novel effector of RhoA/ROCK/myosin II-dependent mechanoadaptation during confined migration and invasive cancer progression.