(500g) Recent Advances in ASD Formulations: Combining Dispersome® Technology with a High-Throughput Screening Setup
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Separations Division
Special Topics in Crystallization: Energy, Sustainability, and Amorphous Precipitation
Tuesday, November 7, 2023 - 1:08pm to 1:26pm
Introduction
Amorphous solid dispersions (ASDs) of poorly water soluble active pharmaceutical ingredients (APIs) in hydrophilic polymeric matrices are a commonly used strategy to increase the solubility and dissolution rate of oral-dosage forms.1 To successfully formulate a growing solubility-impaired pipeline of APIs (i.e. more than 75% of the development pipeline),2,3 recent research efforts on this field have been focused on: i) improving formulation screening methodologies,2 and ii) expand the toolbox with natural carriers with the ability of physically stabilizing amorphous APIs for ASD formulation. β-lactoglobulin (BLG) from whey protein– Dispersome® technology - is a particularly interesting ASD carrier due to the ability of stabilizing amorphous APIs as well as increasing dissolution performance at high loadings.4 Molecular dynamics indicate that stabilization mechanisms are API-load related with hydrogen-bond networks allowing to reach promising stability above 40%.4
This work focuses on two fundamental aspects to obtain optimal ASD formulations: i) high throughput screening (HTS) leveraging Hovione’s proprietary platform ASD-HIPROS and ii) the use of BLG protein (Dispersome®) as an alternative excipient to broad the formulation design-space. A description of the requirements and outputs of a HTS formulation is given, as well as the improvements obtained in specific ASD formulations obtained with Dispersome®.
Methods
In silico formulation and solvent screening based on API molecular descriptors was conducted using computational methods implemented in Python.
Spray dried formulations were obtained using a Buchi B290 and a customized lab-scale unit (24 prototypes per API). Post drying of the spray dried powders performed using vacuum tray ovens until residual solvent levels were below ICH limit.
Final spray dried dispersion (SDD) prototypes characterization encompassed two-stage bio-relevant dissolution and modulated scanning calorimetry (mDSC).
Results and Discussion
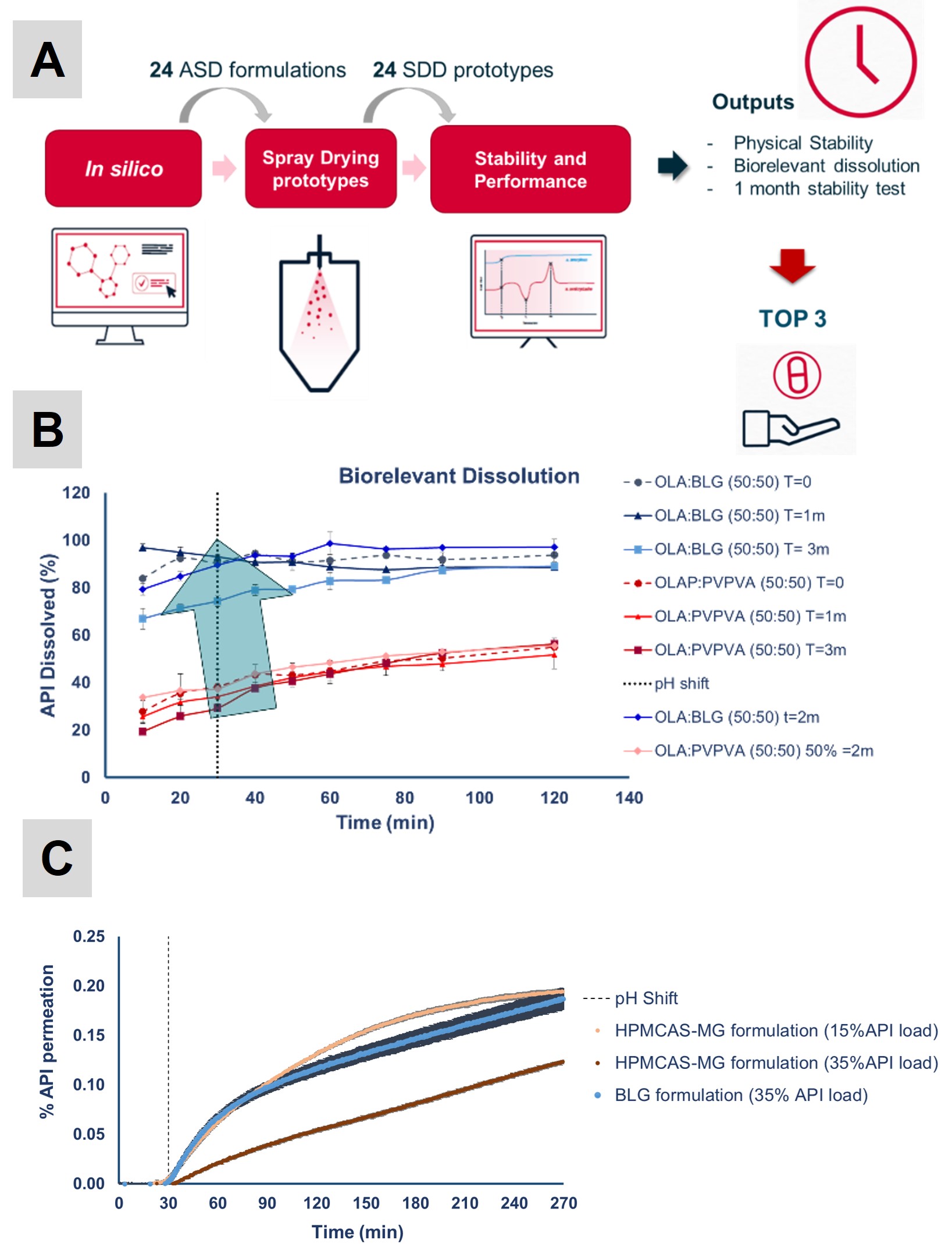
Traditional screening of ASD formulations either requires the use of proxy methods such as i) solvent casting (significant physical and performance differences to spray dried material), or ii) extensive testing in lab SD equipment (time and material consuming). Given the large number of variables in optimizing an ASD formulation (drug load, polymer, surfactant), the number of prototypes to be tested must be reduced. In the HTS platform (ASD-HIPROS – Figure 1A), the first step consists in a computation screening phase based on the Flory-Huggins equation coupled with a drying kinetics and particle formation model. This stage allows the elimination of prototypes that have a high probability of failing and therefore will focus all development efforts on prototypes that are likely viable.
After in silico screening, up to 24 prototypes can be produced and characterized in a single trial – a direct benchmark between BLG and traditional polymers is feasible within a limited timeframe. The number of tested prototypes and the fact that actual spray dried material is produced greatly ensures that an optimal formulation is obtained.
In Figure 1B, a biorelevant dissolution benchmark is presented using BLG and a golden standard polymer PVP-VA 64 to formulate an DCS class IV weak base. PVP-VA 64 provided highest miscibility scores comparing to cellulose- and methacrylate-based polymers and therefore was selected for the study. At 50% API load, BLG allowed to double performance, allowing to increase Cmax from 50% to 100% release in fasted-state intestinal fluid (FaSSIF) as well as to maintain supersaturation for at least 2h. Formulations remained amorphous and chemically stable with equivalent biorelevant dissolution performance after 3M storage under accelerated stability conditions (40ºC/RH75%). Higher API loads and improved permeation performance vs. polymer-based dispersions has been demonstrated (Figure 1C).
Conclusion
ASDs are an established solubility- enhancement platform for DCS class II and IV drugs. Computational methods coupled with high-throughput screening in the streamlined ASD-HIPROS platform can significantly improve ASD screening and reduce development time and effort. Coupled with innovative carriers (eg. Dispersome®), improved formulations (higher performance and drug load) can be developed with minimum time and reduced risk. BLG was demonstrated as a viable carrier for ASDs with the potential to significantly increase both drug load and bioavailability. Hence, Dispersome® technology expands the ASD-HIPROS toolkit allowing for enhanced solubility and increased bioavailability at high drug loadings – demonstrated in-vitro and in-vivo for DCS class II and IV drugs. Further bioavailability enhancement opportunities with better patient compliance for high dosages may be achieved.
REFERENCES
1. Schittny, A., Huwyler, J. & Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: a review. Drug Deliv. 27, 110 (2020).
2. Tambe, S., Jain, D., Meruva, S.K., et al. Recent Advances in Amorphous Solid Dispersions: Preformulation, Formulation Strategies, Technological Advancements and Characterization. Pharmaceutics 14, 2203 (2022).
3. Bennett-Lenane, H., O’Shea, J. P., O’Driscoll, C. M. & Griffin, B. T. A Retrospective Biopharmaceutical Analysis of >800 Approved Oral Drug Products: Are Drug Properties of Solid Dispersions and Lipid-Based Formulations Distinctive? J. Pharm. Sci. 109, 3248 (2020).
4. Kebedev, A.,Donglei, L., Foderà , V. et al. Stabilizing mechanisms of β-lactoglobulin. Mol. Pharmaceutics. 19(11), 3922 (2022).
Figure 1: A) Schematic representation of ASD-HIPROS workflow for high-throughput (HTS) formulation screening. B) Dissolution profiles of a model weakly basic API (DCS class IV). ASDs 50:50 (%w/w) were produced using BLG as carrier and golden standard PVP-VA as benchmark. Two-stage biorelevant dissolution was characterized immediately after secondary drying (T0 – dashed lines) and after 3M at 40ºC/RH75% (closed vial conditions – full lines). A pH shift from FaSSGF medium (pH 1.6) shift to FaSSIF medium (pH 6.5) was conducted after 30 min. Curves correspond to total API released in the dissolution medium, determined by centrifugation (free dissolved drug, micelle-bound drug, and colloids) (as a % of dose)). Results are presented as mean ± SD (n = 2). C) Permeation of model APIs formulated as ASDs using BLG and HPMCAS MG and at 15 and 35% API load. Results are given in % API permeation normalized to dose as mean ±SD, from 4 biological replicates.