(400c) Advances in Optimal, Individualized Drug Dosing Strategies, Based on Hybrid Models and Dynamic Optimization Under Uncertainty
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Computing and Systems Technology Division
Design and Operations Under Uncertainty
Thursday, November 9, 2023 - 4:14pm to 4:36pm
Over the last 15 years, the process systems engineering community has addressed and partially solved some of the aforementioned challenges. For example, Doyle et al. (2014) developed a closed-loop approach to insulin administration (the “artificial pancreasâ€), which relies on physiologically based pharmacokinetic (PBPK) models. NaÈ™cu et al. (2017) devised a closed-loop systems for inducing and maintaining anesthesia, based on PBPK models, and analyzed the impact of inter-patient variability on the results of their calculations. Savoca at al. (2018) studied the transdermal administration of melatonin, using dedicated PBPK models, which include special compartments that model the diffusion of melatonin through the skin. LaiÌnez et al. (2011) proposed general strategies for modeling inter-patient variability, based on Bayesian statistics. And, finally, Hartmanshenn et al. (2018) proposed to improve existing physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) models, by combining omics approaches with the conventional PBPK/PD modeling paradigm. However, despite the substantial progress, made over the last decade, individualized medicine is a research area that still offers significant challenges and opportunities.
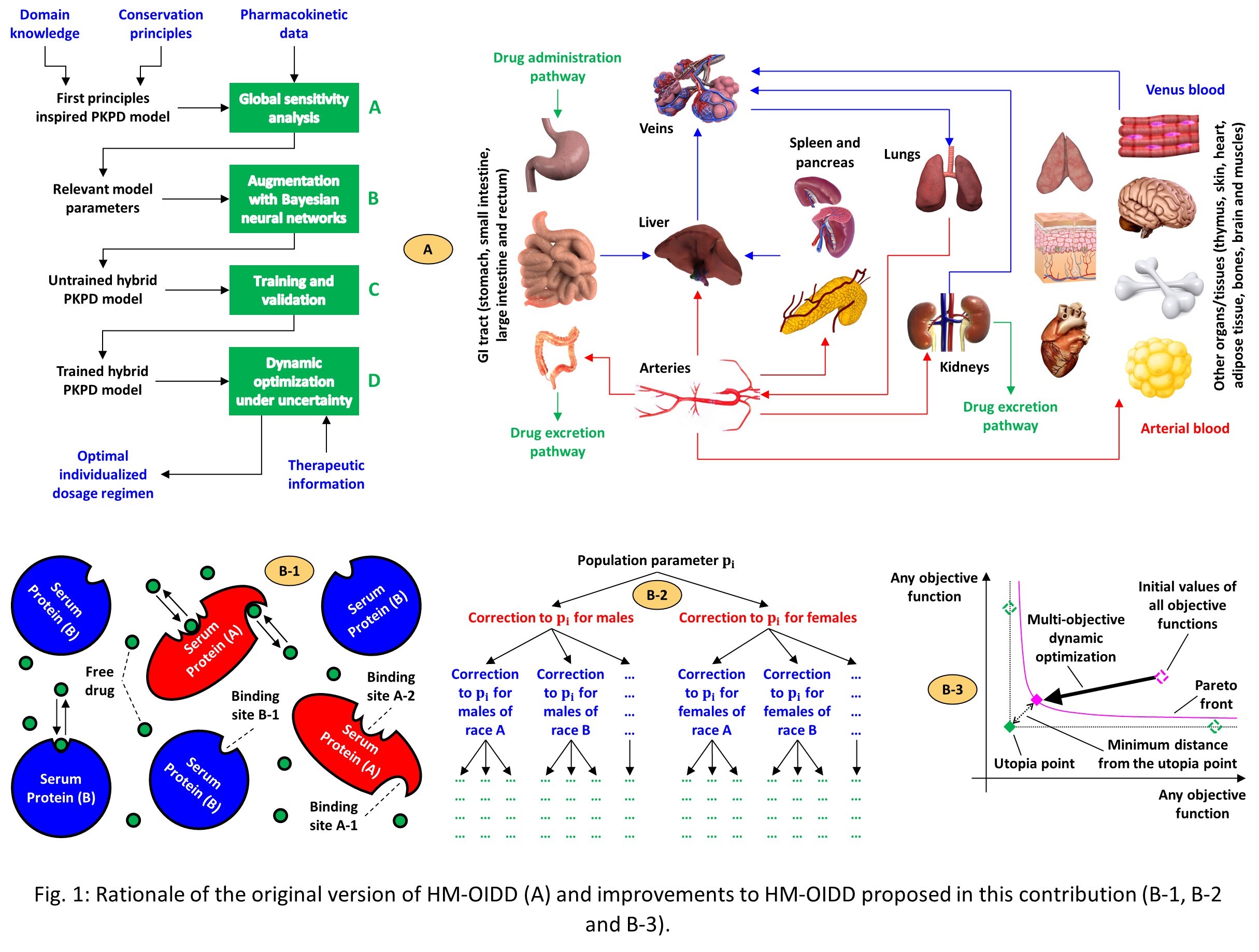
One of the latest attempts at developing a general approach to optimal, individualized drug administration is the framework reported by Rossi et al. (2022) (HM-OIDD), the rationale of which is summarized in Fig. 1-A. This strategy utilizes hybrid, physiologically based pharmacokinetic models, able to predict the patient’s response to treatment with the drug product of interest, complemented with predictions of its degree of uncertainty, in combination with dynamic optimization under uncertainty, to compute optimal drug dosage regimes for each target patient. In addition, it offers dedicated tools for rigorous analysis of the benefits of individualized drug dosing over one-size-first-all approaches. The aforementioned hybrid PBPK models, comprised of a first principles inspired pharmacokinetic component combined with a Bayesian component, are one of the key elements of HM-OIDD, in that they retain the most important benefits of both first principles and machine learning models while also mitigating their most significant disadvantages, at the cost of higher computational complexity. More specifically, the first principles inspired pharmacokinetic component provides physics awareness, enhances model interpretability and extrapolability, and reduces the need for comprehensive, high-quality training datasets. In turn, the Bayesian component allows continued model learning from newly available training data, boosts prediction accuracy, and provides reliable prediction uncertainty estimates. Overall, all these features allow mitigation of most of the challenges which need to be overcome to realize the goal of optimal, individualized drug administration. The second key component of HM-OIDD is the strategy for dynamic optimization under uncertainty on which it relies, which offers the benefits of both robust and stochastic optimization while mitigating the downsides of both. This very unique optimization method, based on the work of Rossi et al., 2016, provides optimal solutions to individualized drug administration problems, which offer an excellent compromise between the need for robustness to inter-patient variability and the desire for high therapeutic efficacy, at reasonable computation cost.
While HM-OIDD already offers an array of very interesting features, it still requires improvement in multiple areas. In this contribution, we propose improvements to both of its two key components, namely, the hybrid PBPK models and the strategy for dynamic optimization under uncertainty. More specifically, we augment the first principles inspired component of the aforementioned hybrid PBPK models with a new drug – serum protein binding model (Fig. 1-B-1), which goes beyond conventional versions thereof that consider serum proteins to be always in large excess and lump all drug – protein interactions into empirical equilibrium constants (CFREE DRUG, PLASMA = KEQ,DRUG∙CTOTAL DRUG, PLASMA). The proposed, new drug – serum protein binding model is indeed able to represent the individual interactions between drug molecules and different types of serum proteins, and also allows for multiple binding sites per protein type. In addition, we also convert the non-hierarchical Bayesian component, currently embedded in the aforementioned hybrid PBPK models, which can only operate on the entire patient population as a whole, into a hierarchical version thereof (Fig. 1-B-2), which operates on appropriate patient sub-groups with similar characteristics (patients are organized in a tree-like fashion by gender, race, and, possibly, other relevant covariates), so as to increase prediction accuracy and reduce prediction uncertainty. This new hierarchical Bayesian component will also incorporate ad-hoc tools for dimensionality reduction, such as dedicated strategies for identification of those hybrid model parameters that can be fixed to their maximum likelihood estimates, so as to ensure that the overall computational complexity of the resulting hybrid PBPK models is still compatible with dynamic optimization calculations under uncertainty. Finally, we augment the strategy for dynamic optimization under uncertainty, included in HM-OIDD, by adding new types of objective functions (for example, the time over which the plasma drug concentration lies outside of the therapeutic range) and by allowing automatic formulation and solution of multi-objective individualized drug dosing problems, in which multiple contrasting objectives are considered simultaneously (Fig. 1-B-3).
The impact of these advances is demonstrated on a comprehensive case study, based on the drug Vancomycin (Vancomycin is a potent antibiotic, used to treat serious Gram-positive bacterial infections, which can cause severe adverse effects, including permanent organ damage, if not properly administered). In particular, we first utilize the extended version of HM-OIDD, proposed in this manuscript, to compute optimal Vancomycin dosing regimens for a comprehensive cohort of in-silico patients, featuring different genders, ages, body mass indices, serum albumin levels (hypoalbuminemia may be common in patients undergoing certain types of medical treatments, such as chemotherapy), and degrees of renal function (in this study, we assume that degree of renal function and serum creatinine levels are directly correlated), and then compare the results obtained to those offered by the original version of HM-OIDD. The results of this case study are encouraging, in that the extended version of HM-OIDD, proposed in this contribution, outperforms the original version of HM-OIDD in terms of application domain, efficacy, and safety.
As a final remark, note that all the strategies for hybrid modeling and dynamic optimization under uncertainty, included in HM-OIDD, target individualized medicine applications. However, the same types of strategies can be applied to many other types of design, optimization, and condition monitoring problems, subject to considerable model and data uncertainties. The latter include most engineering problems in pharmaceutical, bio-pharmaceutical and fine chemicals manufacturing, food processing/production, and defense.
References
Doyle III, F. J., Huyett, L. M., Lee, J. B., Zisser, H. C., & Dassau, E. (2014). Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes care, 37, 1191-1197.
Hartmanshenn, C., Rao, R. T., Bae, S. A., Scherholz, M. L., Acevedo, A., Pierre, K. K., & Androulakis, I. P. (2018). Quantitative systems pharmacology: Extending the envelope through systems engineering. Computer Aided Chemical Engineering, 42, 3-34.
LaiÌnez, J. M., Blau, G., Mockus, L., Orçun, S., & Reklaitis, G. V. (2011). Pharmacokinetic based design of individualized dosage regimens using a Bayesian approach. Industrial & Engineering Chemistry Research, 50, 5114-5130.
Nașcu, I., Oberdieck, R., & Pistikopoulos, E. N. (2017). Explicit hybrid model predictive control strategies for intravenous anaesthesia. Computers & Chemical Engineering, 106, 814-825.
Savoca, A., Mistraletti, G., & Manca, D. (2018). A physiologically-based diffusion-compartment model for transdermal administration–The melatonin case study. Computers & Chemical Engineering, 113, 115-124.
Rossi, F., Mockus, L., Nagy, Z., & Reklaitis, G. (2022). A general strategy for optimal, individualized drug dosing, based on hybrid models and dynamic optimization under uncertainty. Paper 688f, 2022 AIChE Annual Meeting, Phoenix, AZ.
Rossi, F., Reklaitis, G., Manenti, F., & Buzzi-Ferraris, G. (2016). Multi-scenario robust online optimization and control of fed-batch systems via dynamic model-based scenario selection. AIChE Journal, 62, 3264-3284.