(331b) Tunable and Scalable Immobilization of Oligo-Dt Ligands on Membranes for mRNA Capture-By-Design
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Separations Division
Frontiers in New Materials and Membranes for Bioseparations
Tuesday, November 7, 2023 - 8:40am to 9:00am
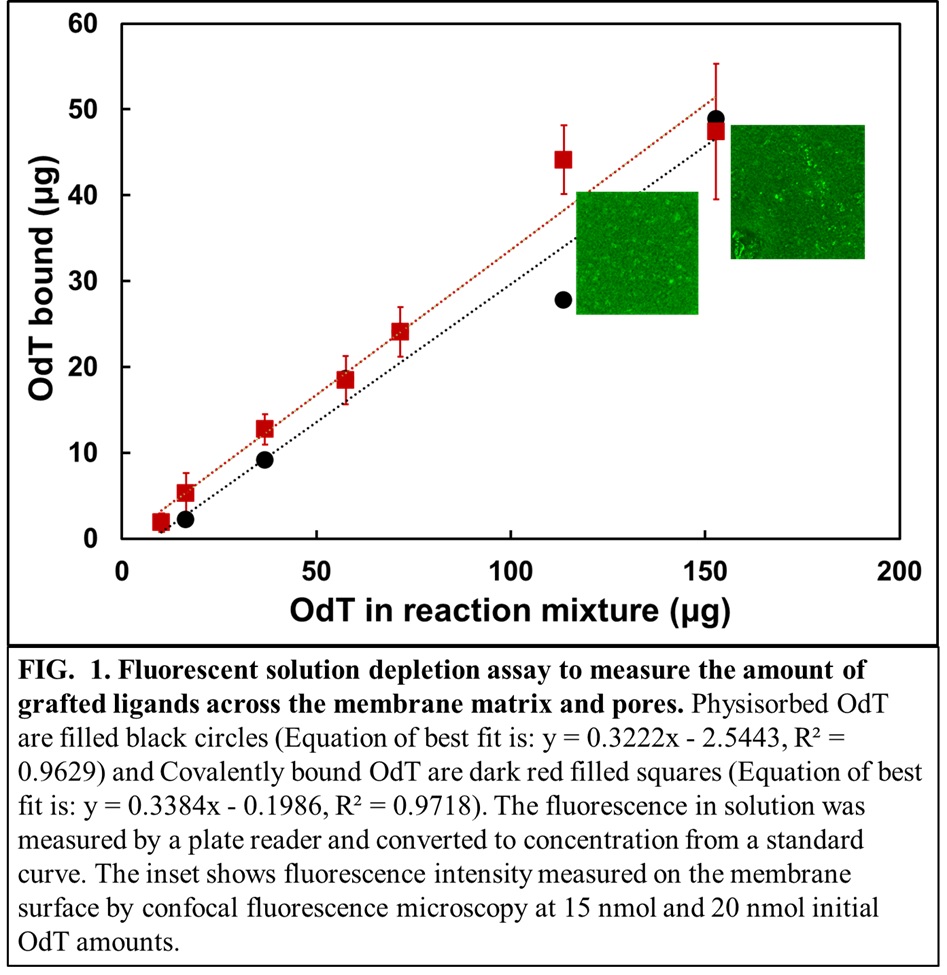
Membranes have several fundamental advantages over chromatographic resins like shorter residence times, less equipment footprint and scalability. In this work, oligo-dT was immobilized on microporous regenerated cellulose (RC) membranes in flat sheet and hollow fiber configurations. The reaction systems were designed to improve scalability and tunability of the grafted ligands. The parameters involved for efficient scale-up of the single electron transfer-living radical polymerization (SET-LRP) reaction for covalently grafting acrylate labelled poly-T were identified. The quantification of the amount of oligo-dT covalently grafted on the RC membranes used a solution-fluorescence-depletion assay when the grafted ligand had a FAM fluorophore (a single isomer derivative of fluorescein) attached to it (Fig.1).
The oligo-dTs were immobilized by two different grafting techniques, one involving a single step reaction with an acrylate modified oligo-dT and the other involving a two-step reaction of grafting a dibenzocyclooctyne acrylate followed by a strain induced click reaction with an azide labelled oligo-dT. We hypothesized that the grafting of smaller molecules with SET-LRP reaction will result in less steric hindrance and reduced amount of unreacted oligos during grafting. The extent of control of the grafted layer depended on the choice of reaction mechanism, where the one-step method can be chosen as a faster or more economical alternative, the two-step method can give better specificity and distribution of grafted oligo-dTs with the click reaction being independent of catalyst surface contact. The 2 methods were compared for optimum degree of grafting with the highest binding and elution of fluc-mRNA under the chosen buffer conditions.
An estimation of the distribution of grafted oligo-dT ligands across the membrane matrix and pores is essential to understand the effects of steric hindrance while binding a target mRNA. To evaluate this, a 3D voxel analysis of an RC membrane grafted with FAM labelled oligo dT was performed that provides highly sensitive details of the distribution of immobilized ligands across a layer of membrane thickness (Inserts in Fig.1). A library of oligo-dTs varying in length, spacer lengths and the sequence of nucleotides was studied for binding with oligo-dA and Firefly Luciferase (fluc) mRNA of ~1900 nt. The binding of oligo-dTs of different architecture to oligo-dAs of different lengths will be illustrated and the rules for choosing the optimum length, spacer and sequence of oligo dT will be set. Surface characterization data with fluorescence microscopy will be correlated with measurements of binding and unbinding and these results will define the most important parameters that influence the oligo-dT ligand architecture for all affinity-based systems. The scaleup criteria that results from these experiments will be useful to design commercial membrane modules for fast and efficient purification of intact mRNA drug products in terms of all secondary structures.