(238d) Solvent Selection for Desired Crystal Form during Industrial Crystallization: A Solution Thermodynamics Study
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Separations Division
Crystallization and Precipitation of Pharmaceutical and Biological Molecules
Wednesday, November 8, 2023 - 1:27pm to 1:45pm
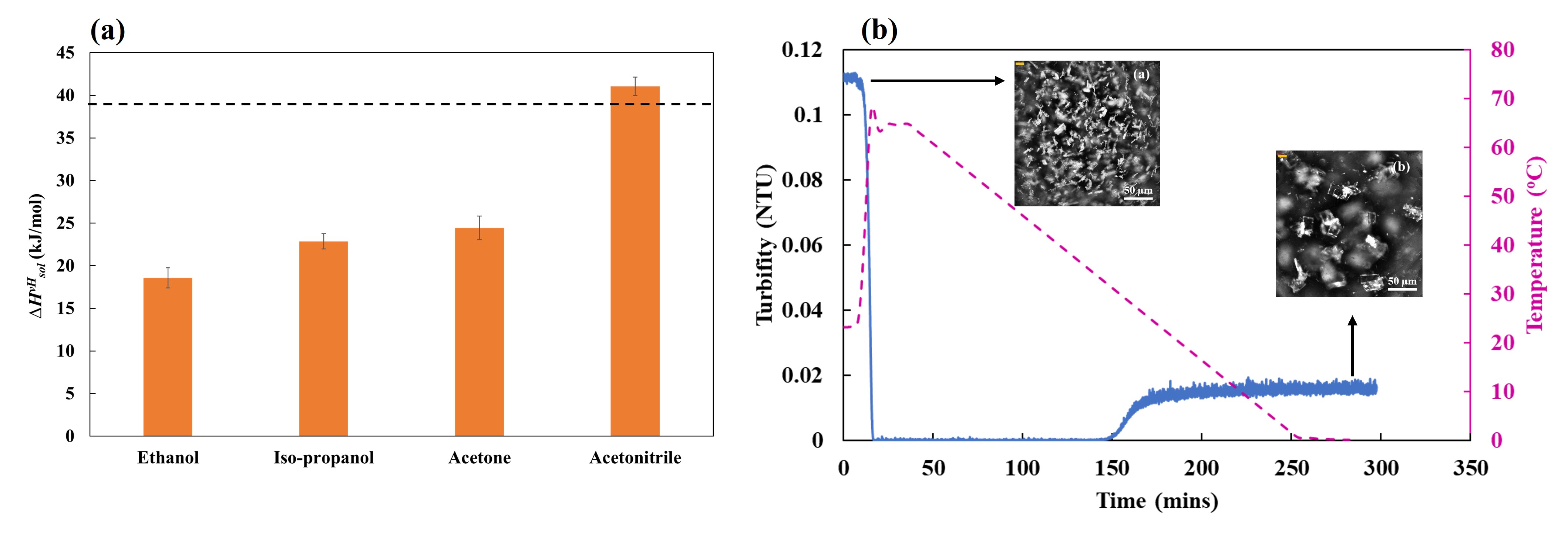
Solvents play a primary role during pharmaceutical crystallization by influencing the polymorphic outcome. Various solvent-selection strategies have been explored to help identify a solvent system from which the desired drug form can be crystallized. The work described here investigated the use of solution thermodynamics to obtain descriptors that can aid solvent screening and process development at lab scale. BMS-817399 has been used as a model compound that exists as a hydrate (Form 1), but an anhydrous form is desired for drug formulation. Solubility data obtained from preliminary crystallization experiments conducted at small scale (1 mL) in different solvents (ethanol, iso-propanol, acetone, and acetonitrile) were modeled using the van’t Hoff framework to derive solution properties such as van’t Hoff enthalpy of solution (). It was observed that the value of in acetonitrile was almost three times higher than in other solvents (Fig. 1a). Furthermore, the activity coefficient (g) of the solute in acetonitrile was estimated to be much greater than 1, implying that solute-solute interactions in solution are stronger than the solute-solvent interactions. Thus, acetonitrile was chosen to crystallize the compound at lab-scale (100 mL). A novel anhydrous polymorph (Form 2) was discovered during crystallization from acetonitrile (Fig. 1b); the new polymorph exhibited higher stability to varied relative humidities, making it an attractive candidate for drug formulations. The current methodology can be especially useful for drug compounds that are still in the development stage, and hence are scarcely available during the initial stages of drug development.