(230g) Surface Growth of Soot By Acetylene Pyrolysis Using Reactive Molecular Dynamics
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Particle Technology Forum
Particle Engineering Applications: Additive Manufacturing, Energy Storage and Carbon Capture
Tuesday, November 7, 2023 - 5:30pm to 5:50pm
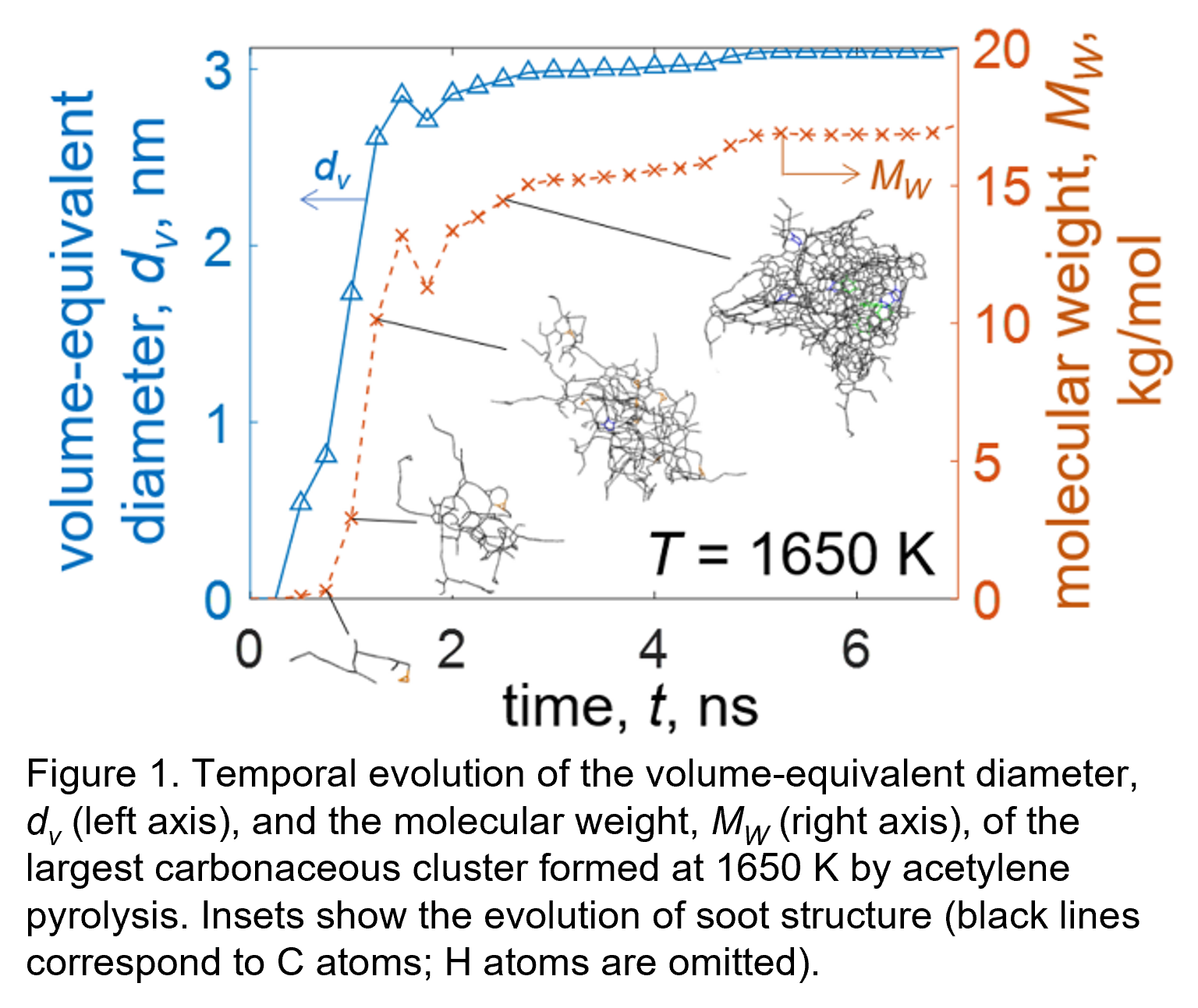
Here full-atom reactive molecular dynamics (MD) simulations are employed to capture the dynamics of incipient soot formation and growth by acetylene pyrolysis. The growth of the soot nanoparticles is monitored to derive a precise reaction rate constant accounting for soot cluster morphology, aromaticity, and composition during its growth. Figure 1 shows the temporal evolution of the volume-equivalent diameter, dv (left axis), and the molecular weight, MW (right axis), of the largest carbonaceous cluster formed at each timestep at 1650 K. Insets show snapshots of the largest cluster as a function time, indicating the formation an amorphous shell consisting of aliphatic chains and a core consisting of aliphatic and aromatic ring structures. Furthermore, the effect of process conditions (e.g., temperature, particle number concentration) on soot dynamics is quantified. The MD-obtained temperature-dependent reaction rate constant is interfaced with a monodisperse particle dynamics model (Kelesidis & Kholghy, 2021) to predict the soot volume fraction, finding excellent agreement with measurements in premixed ethylene flames.
MD simulations are used to track the growth of soot clusters formed by acetylene pyrolysis. The effect of temperature on the structure, composition, and morphology of soot as well as on its growth rate is quantified. The robust MD-obtained reaction rate derived here can be interfaced with a monodisperse particle model to elucidate the growth of soot in fractal-aggregates and thus simulate accurately the evolution of the soot volume fraction.
Mathieu, O., Djebaïli-Chaumeix, N., Paillard, C. E., & Douce, F. (2009). Combustion and Flame, 156(8), 1576-1586.
Mosbach, S., Celnik, M. S., Raj, A., Kraft, M., Zhang, H. R., Kubo, S., & Kim, K. O. (2009). Combustion and Flame, 156(6), 1156-1165.
Kelesidis, G. A., & Kholghy, M. R. (2021). Materials, 14(14), 3882.
Ramanathan, V., & Carmichael, G. (2008). Nature geoscience, 1(4), 221-227.