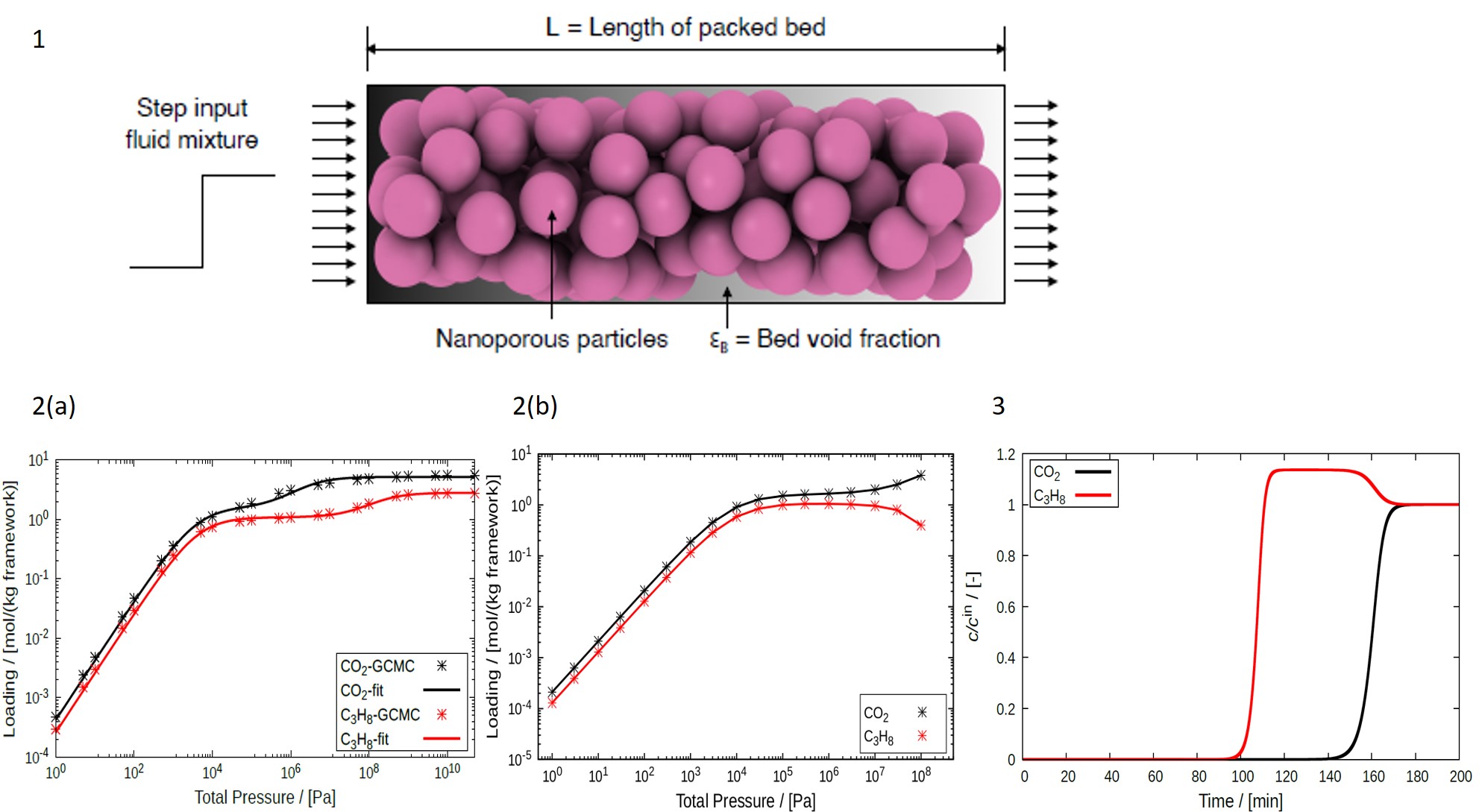
Adsorption plays an important role in separation and purification of fluid mixtures. Estimation of adsorption isotherms are crucial in designing adsorption-based processes. These isotherms quantify the affinity between the fluid phase molecules (adsorbate) and the solid phase (adsorbent). Many industrial separation processes take place at dynamic conditions. In such a system, the concentration of the fluid phase components measured at the outlet of the adsorption column (Fig. 1) as a function of time are known as breakthrough curves. Adsorption isotherms (Fig. 2) and breakthrough curves (Fig. 3) can be obtained experimentally but this process is very time consuming as many experiments are required to generate the datasets. Therefore, one can always opt for modelling and simulation-based estimation of these curves. Acknowledging the importance of the adsorption isotherms and breakthrough curves calls for the need of open-source software which can calculate these curves. Hereby, we present RUPTURA code (
https://github.com/iraspa/ruptura) as a free and open-source software package (MIT license) [1]. RUPTURA is capable of (1) simulating breakthrough curves for the adsorption of gas phase molecules at isothermal conditions (Fig. 3), (2) predicting mixture isotherms (Fig. 2b) using methods like the Ideal Adsorption Solution Theory (IAST), Segregated-IAST (SIAST), and explicit isotherm models, and (3) fitting of pure-component isotherm (Fig. 2a) models on computed or experimentally measured pure component adsorption isotherm data. For breakthrough curve simulations, RUPTURA supports both step and pulse style input for the feed mixture. This code also includes a wide variety of pure-component isotherm models like Langmuir, Anti-Langmuir, BET, Henry, Freundlich, Sips, Langmuir-Freundlich, Redlich- Peterson, Toth, Unilan, O’Brian & Myers, and Asymptotic Temkin. Mixture prediction and breakthrough simulations rely on these isotherm models that are obtained by fitting isotherm data. In RUPTURA, fitting of pure-component isotherms is performed using genetic algorithm. Breakthrough plots and animations depicting the variation of properties like fluid velocity, column pressure, and adsorbed loadings along the fixed bed are automatically generated. We foresee our code being used in (industrial) research for screening adsorbents for separation processes based on Pressure Swing Adsorption (PSA), and Temperature Swing Adsorption (TSA) but also for teaching in chemistry and chemical engineering classes.
[1] S. Sharma, S.R.G. Balestra, R. Baur, U. Agarwal, E. Zuidema, M.S. Rigutto, S. Calero, T.J.H. Vlugt, and D. Dubbeldam. RUPTURA: Simulation Code for Breakthrough, Ideal Adsorption Solution Theory Computations, and Fitting of Isotherm Models. Mol. Sim., 2023. Under review.
Captions to Figures
Figure 1. Schematic illustration of a fixed bed adsorber column. The cylindrical column is filled with micro/ nano porous adsorbent material (crystalline or amorphous). When a fluid mixture passes through the column, adsorption of different components present in the mixture takes place inside the adsorbent materials.
Figure 2. Adsorption isotherms for CO2 and C3H8 in MOR-type zeolite at 300K. (a) Pure-component isotherms obtained by fitting dual-site Langmuir isotherm model to pure component adsorbed loadings computed using molecular simulations. (b) Mixture isotherms for an equimolar mixture of CO2 and C3H8 (50%:50%) using Segregated Ideal Adsorbed Solution Theory (SIAST).
Figure 3. Breakthrough curves obtained using RUPTURA for a mixture consisting of 10% CO2, 10% C3H8, and 80% Helium in MOR-type zeolite at 300K and 1bar pressure. The adsorption column is operated in isothermal and isobaric conditions. The adsorption of C3H8 is weaker compared to CO2. Therefore, C3H8 exits the column first and shows a roll-up behaviour. This roll-up behaviour occurs when the gas phase concentration at the outlet for a certain component exceeds the inlet concentration.