(174d) High Throughput Testing Strategies for Alkali Activation of Cementitious Mixture
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Materials Engineering and Sciences Division
Accelerated Discovery of Inorganic Materials: High-Throughput Experiments, Modeling, and Data Science
Thursday, November 9, 2023 - 1:30pm to 1:45pm
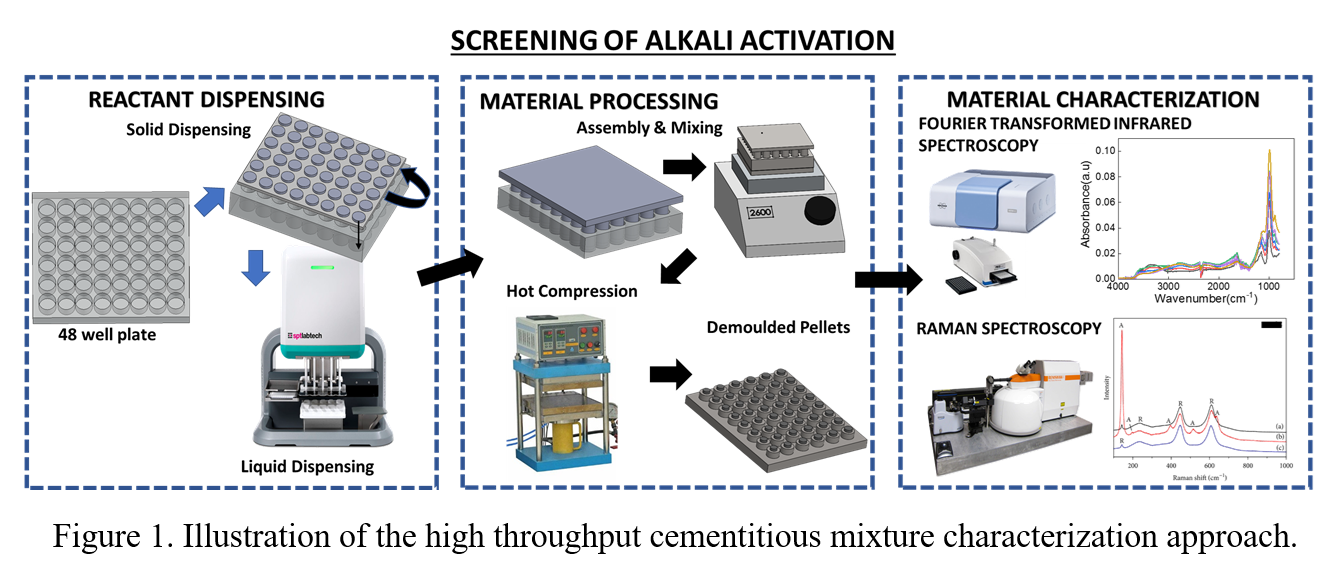
Geopolymer concrete can be produced from industrial mineral waste like coal ash, slag, and different sources (different production facilities and landfills). This geopolymer concrete can be exactly used as traditional Portland cement. The major challenge in these geopolymer production processes is the variability in feedstock composition. Due to this challenge, the process parameters (such as alkali concentration and S/L) must be changed for every feedstock. In this work, we developed a high throughput strategy to evaluate and optimize the alkali activation process for the cementitious mixture (i.e., carbonated industrial mineral waste) to form geopolymer concrete. This method will rapidly screen the process parameters. Figure 1. shows a three-step process: automated dispensing of carbonated residues and liquid (Alkali activator), reaction activation, and gel characterization. To obtain a statistically relevant size of cement, we designed and fabricated microtiter plates ( one with 36 wells and the other with 48 wells) of ~ 1 cm well diameter. A solid dispenser that was designed and printed using a 3d printer was used to dispense a fixed amount (~50 mg to 100 mg) of carbonated residue. A robotic liquid dispenser (Dragonfly Discovery) was used to add a liquid solution (~ 0.01 ml to 0.1 ml) of specific composition to the wells. This mixture is mixed well with a vibrator and compacted under high pressure at various temperatures. This study was performed to screen the following range of reaction conditions- (1) various alkali activators ( NaOH, Na2SiO3, KOH, K2SiO3), (2) liquid to solid wt %, (3) Pressure (500-3000 psi), and (4) temperature (5o C-200o C). In this process, the liquid and powder react to form a gel. We characterized it with IR and Raman spectrometers to focus on aluminosilicate network polymerization. The alkali activation process can be observed by shifting the main peak from 1072 cm-1 to 972 cm-1. This change is expected due to the decrease of tetrahedral Si sites, replaced by the tetrahedral Al during the formation of N-A-S-H gel. Raman spectra showed the shift of 667 cm-1 (symmetrical bending Si-O-Si) is sensitive to Al/Si ratio. Furthermore, there are other bands that can confirm the presence of gel, such as 1009 cm-1 (v1 SiO4 symmetrical stretching), 850 cm-1 (v1 SIO4 symmetrical stretching), 488 cm-1 (v4 SiO4) and 444 cm-1 (v2 SiO4 non-bridging Onon-Si-Onon). Currently, we are trying to correlate the spectroscopy signatures to reactivity quantified by calorimetry, thermogravimetry, and eventually mechanical performance. Once validated, this HT screening approach will be used to supply data for ML, which is expected to drastically accelerate mixture development for on-traditional concrete.