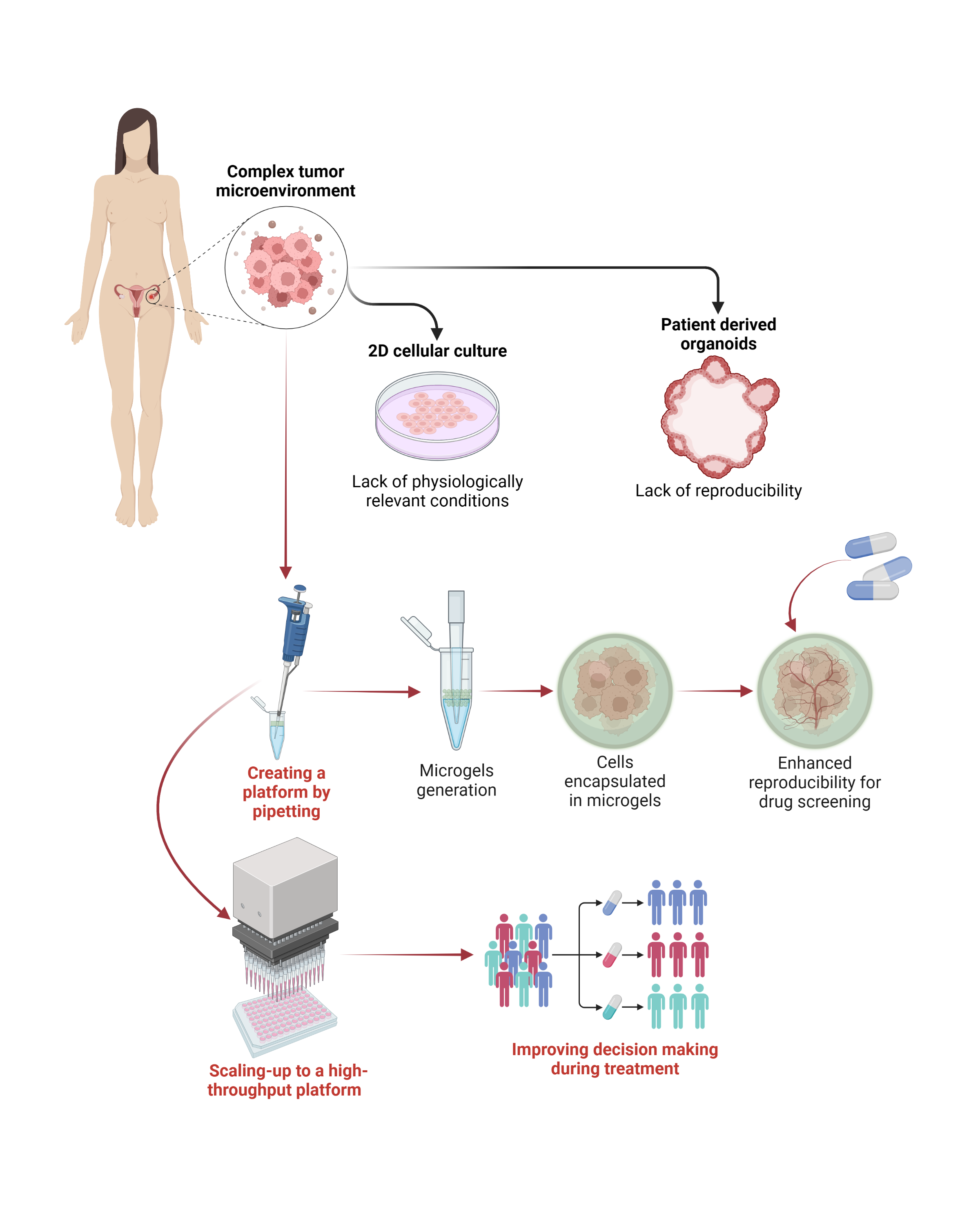
(100b) Development of a High-Throughput Drug Screening Platform Via Pipetting Gel Droplet Micro-Organoids Models
AIChE Annual Meeting
2023
2023 AIChE Annual Meeting
Food, Pharmaceutical & Bioengineering Division
3D Culture: Organoids and Spheroids
Monday, November 6, 2023 - 8:36am to 8:54am
To address this issue, we have developed a home-made pipette microfluidic device for the high-quantity generation of cancer micro-organoids via cell encapsulation by droplet emulsion for high-throughput drug screening. We synthesized norbornene-modified hyaluronic acid (NorHA) polymers, which serve as extracellular matrix (ECM) mimics, to create a suitable cell-encapsulation platform. The viscosity of the polymer solution was adjusted to enable its extrusion by a home-made elliptical pipette for droplet generation with suspended ovarian (OVCAR 5) and prostate (LNCaP) cancer cells (4x106 cells/mL) via water-in-oil (W/O) emulsification.
We achieved the rapid generation of thousands of monodispersed droplets ranging from 370 µm to 420 µm via covalent crosslinking by thiol-Nor complexes, which allowed for further emulsion breaking and separation of gel droplets with ~80% separation efficiency. RGD motifs were added to the gel formulation to improve cell adhesion, while a combination of DTT and MMP-sensitive motifs was used as crosslinkers to control cell proliferation rate. Cell viability assays were conducted upon droplet separation, which exhibited little effects of the encapsulation process on the live/dead ratio. ViaFluor staining and mitochondrial activity quantification through WST-1 were performed to study cell proliferation within the microgels. Confocal microscopy was used to assess the volumetric change of the multicellular constructs, and the permeability of the microgels was evaluated through TRITC-labeled dextran (4400 KDa) to determine the drugs' diffusion capacity through the gels, which showed a significant internalization and accumulation of the fluorophore at 30 min and 60 min, respectively. Finally, screening with doxorubicin (DOX) was used to model the sensitivity of the micro-organoids towards therapies administration.
Overall, the developed microgels platform showed suitability for the encapsulation of different cancer cell lines and organoids' growth, which would allow the screening of multiple tumor microenvironment conditions. The versatility of the technology allows it to be scaled up with multi-channel and/or robotic systems and integrated with flow cytometry to realize a high-throughput platform for patient-derived micro-organoids generation and drug screening.