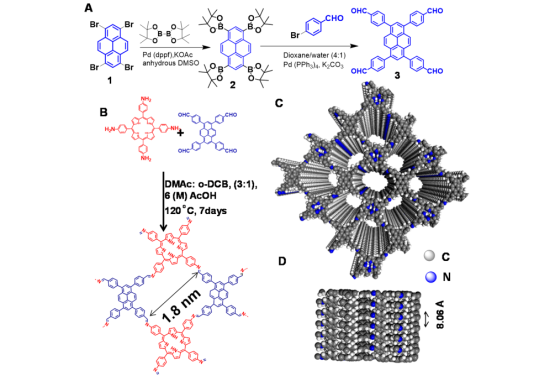
Figure: (A) Synthesis of 1,3,6,8-tetrakis(4-formylphenyl) pyrene (TFFPy). (B) Synthetic scheme of the imine based COF (SB-PORPy). (C) Top view and (D) side view of AA (eclipsed) stacking of SB-PORPy COF (gray, carbon; blue, nitrogen).
1. Synthesis of 1,3,6,8-Tetrakis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene (Py-bpin4):
Materials:1,3,6,8-tetrabromopyrene 1, bis(pinacolato)diboron, Pd(dppf)Cl2, potassium acetate, anhydrous DMSO, Dichloromethane (DCM), Silica gel (60-120 mesh),Toluene.
Equipment: Schlenk tube, Stirrer, Heating mantle, NMR spectrometer, ESI HRMS, FTIR spectrometer
Method:
- In a Schlenk tube, add 1.5 g of 1,3,6,8-tetrabromopyrene 1 (2.9 mmol), bis(pinacolato)diboron (4.4 g, 17.35 mmol), Pd(dppf)Cl2 (0.175 g, 0.25 mmol), and potassium acetate (1.75 g, 17.85 mmol) in 15 mL of anhydrous DMSO.
- Backfill the mixture with N2 three times.
- Heat the reaction mixture at 90 °C for 48 h under stirring condition.
- Cool the reaction mixture to room temperature.
- Extract the mixture with Dichloromethane (DCM).
- Isolate the crude product by solvent evaporation as a yellow solid.
- Purify the crude product by using flash column chromatography on silica gel (60-120 mesh) using dichloromethane/toluene as an eluent.
- Isolate the tetra borylated product 2 as gray solid (1.5 g, yield 76%).
- Measure the melting point (Mp) of the product (>300 °C).
- Analyze the product using NMR spectroscopy, ESI HRMS and FTIR spectroscopy
In summary, the synthesis of 1,3,6,8-Tetrakis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene (Py-bpin4) involves the condensation of 1,3,6,8-tetrabromopyrene 1 with bis(pinacolato)diboron in the presence of Pd(dppf)Cl2 and potassium acetate in anhydrous DMSO. The crude product is then isolated and purified using flash column chromatography on silica gel. The final product is a tetra borylated product 2, which is analyzed using various spectroscopic techniques such as NMR, ESI HRMS
Synthesis of the SB-PORPy-COF:
The materials and equipment needed for the synthesis include a Schlenk tube (50 mL), 5,10,15,20-Tetrakis(4-aminophenyl)porphyrin, 1,3,6,8-tetrakis(4-formylphenyl) pyrene, an acetic-acid catalyst (6 M), o-dichlorobenzene, dimethylacetamide, an oven, and a centrifuge.
- Preparation of the mixture: A mixture of 5,10,15,20-Tetrakis(4-aminophenyl)porphyrin (0.1 mmol, 67 mg) and 1,3,6,8-tetrakis(4-formylphenyl) pyrene (0.1 mmol, 61 mg) was prepared in a Schlenk tube (50 mL) using an acetic-acid catalyst (6 M, 0.5 mL).
- Addition of DMAc/o-DCB mixture: An o-dichlorobenzene (o-DCB)/dimethylacetamide (DMAc) mixture (3.0/1.0 mL) was added to the mixture in the Schlenk tube.
- Sonication: The mixture was sonicated for 1 hour to ensure thorough mixing.
- Degassing and sealing: The tube was degassed via three freeze−pump−thaw cycles to remove any air present. After degassing, the tube was flame-sealed under vacuum to create an airtight environment.
- Heating: The sealed Schlenk tube was heated statically in an oven at 120 °C for 7−8 days to allow for the reaction to occur.
- Collection: The greenish-brown precipitate was collected via centrifugation and washed several times with THF to remove any trapped guest molecules.
- Drying: The powder was collected and dried at 120 °C under vacuum overnight to produce SB-PORPy-COF in an isolated yield of 76%.
Catalyst Preparation:
Materials:
- Vulcan (carbon support)
- SB-PORPy-COF composite catalyst
- Isopropyl alcohol (IPA)
- Distilled water
- Nafion binder (5 wt %)
- Alumina slurry (0.05 μm)
Equipment:
- Mortar and pestle (for grinding the catalyst with Vulcan)
- Vortex mixer or ultrasonic bath (for dispersing the catalyst in the solvent)
- GC electrode (3 mm diameter)
- Syringe (for drop-casting the catalyst ink onto the GC electrode)
- Oven (for drying the electrode)
- Polishing cloth or pad (for polishing the electrode with alumina slurry)
- Distilled water (for washing the electrode)
The next step in the experimental procedure is the preparation of the catalyst using the previously synthesized SB-PORPy-COF material. The catalyst is prepared by mechanical grinding of Vulcan, a carbon support material, with the SB-PORPy-COF in a 1:1 weight ratio.
To prepare the catalyst ink, 2 mg of the composite catalyst is dispersed in 1 mL of a mixed solvent solution consisting of isopropyl alcohol (IPA) and water in a 1:1 volume ratio. Additionally, 20 μL of a 0.5 wt% nafion binder is added to the solution to aid in the dispersion of the catalyst particles and to improve its adhesion to the electrode surface. The nafion binder is first diluted from 5 wt% to 0.5 wt% with IPA.
After the catalyst ink is prepared, a 10 μL droplet of it is deposited on a GC electrode (3 mm diameter) and dried overnight in air. Before depositing the catalyst, the GC electrode is polished with 0.05 μm alumina slurry and washed several times with distilled water. This helps to ensure a clean and uniform surface for the catalyst deposition.
References:
1. Bhunia, Subhajit; Das, Sabuj Kanti; Jana, Rajkumar; Peter, Sebastian C.; Bhattacharya, Santanu; Addicoat, Matthew; Bhaumik, Asim; Pradhan, Anirban (2017). Electrochemical Stimuli-Driven Facile Metal-Free Hydrogen Evolution from Pyrene-Porphyrin-Based Crystalline Covalent Organic Framework. ACS Applied Materials & Interfaces, (), acsami.7b06968–. doi:10.1021/acsami.7b06968

- Log in to post comments
