(708a) Oxidative Coupling of Methane (OCM) over Strontium-Doped Neodymium Oxide: Parametric Evaluations and the Effect of Water Addition
AIChE Annual Meeting
2022
2022 Annual Meeting
Catalysis and Reaction Engineering Division
Hydrocarbon Conversion II: Oxidative processes for hydrocarbon conversion
Friday, November 18, 2022 - 12:30pm to 12:48pm
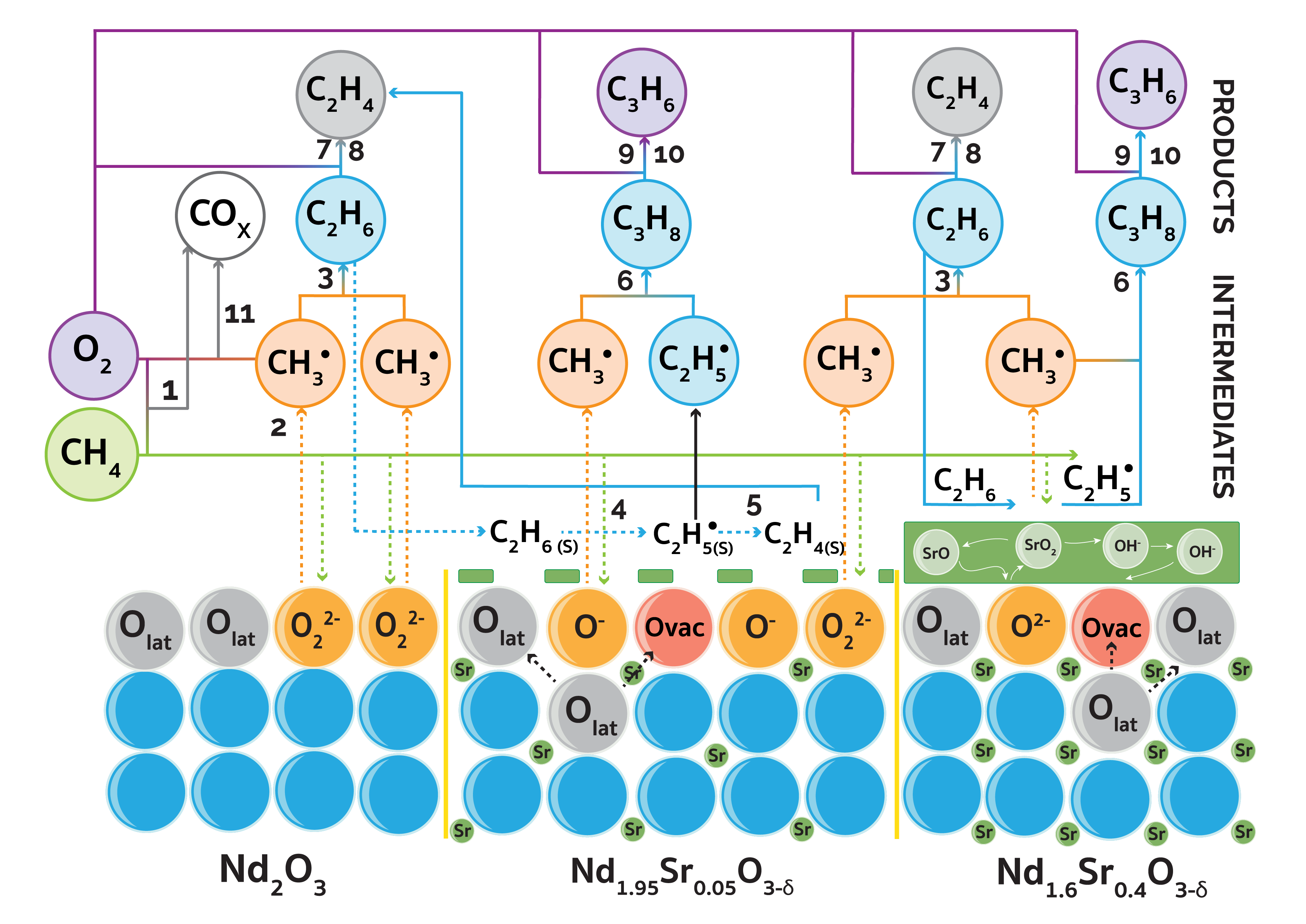
Since its discovery in 1982, oxidative coupling of methane (OCM) has been considered one of the most promising approaches for the on-purpose synthesis of ethylene. As is the case with many oxidation processes, however, OCM may produce undesirable side products. Hence, the development of more selective catalysts is essential to positive process economics; and alkaline earth doped rare earth metal oxides have shown significant promise in maintaining high C2+ selectivity. In this work, undoped neodymium oxide as well as neodymium oxide doped with high and low levels of strontium were tested in high-throughput OCM experiments covering wide range of operating conditions including temperatures of 700-800 °C, methane to oxygen feed ratios of 3.5-13 and residence times of 10-50 milliseconds. Strontium doping improved C2+ yield in all the temperature and feed ratio ranges; and the catalysts were shown to be able to achieve greater than 18% C2+ yield. Space velocity was also shown to play a significant role in C2+ selectivity. For a methane to oxygen feed ratio of 3.5, selectivity increased with increasing space velocity reaching a maximum of 62% at a methane conversion of 30% and producing an optimal space velocity of ~250,000 mL/h/g. Moreover, since methane conversion was limited by available molecular oxygen concentration due to safety limitations, it was found that this could be partially compensated for by co-feeding water, which produced stable operation with high C2+ selectivity. The difference in activity between the three samples was linked to the contribution of different oxygen centers; neodymium peroxide anions in the case of pristine neodymium oxide and mixtures of peroxide and anionic O- species for Nd1.95Sr0.05O3-δ, and strontium peroxide assisted by previously mentioned oxygen centers for Nd1.6Sr0.4O3-δ as illustrated in the figure below.