(589c) Concentration Gradient Generation Using Spatially Non-Uniform AC Electric Fields
AIChE Annual Meeting
2022
2022 Annual Meeting
Engineering Sciences and Fundamentals
Interfacial Phenomena in Electrochemical and Electrokinetic Systems
Thursday, November 17, 2022 - 8:30am to 8:45am
Spatial and temporal gradients are essential in biological systems playing important roles in cell signaling, migration, differentiation, and metastasis. Conventional gradient-generation platforms are based predominantly on diffusion, printing, dip coating, or irradiation. However, most of these techniques only produce static, monotonic gradients that have fixed physiochemical properties, whereas studying dynamic biomolecular responses requires spatially and temporally controllable gradients. Microfluidic platforms have been used to generate passive chemical gradients which feature spatial and temporal control. Electrochemical techniques are able to actively create dynamic surface gradients that feature compelling controllability and flexibility. Additionally, electrochemical gradient generation techniques are highly versatile, are compatible with organic and inorganic systems, can be integrated with electronics, and are easily automated. However, most of the current electrochemical gradient generation approaches rely on Faradaic reactions that yield species accumulation for gradient generation. We hypothesized that stable concentration gradients can be generated using biased electromigration in spatially non-uniform alternating-current (AC) electric fields independently from bulk flux and without Faradaic reactions. The fluorescent emission intensity changes detected in the remaining experiments could be decoupled and attributable to electromigration mechanisms. Spatial and temporal analysis on the intensity behavior were performed and visually tabulated the ion concentration gradients using contour plots.
Materials and Methods
In this exploratory study, we have used ionized fluorescent dye Fluorescein isothiocyanate (FITC), was used as a real-time detectable target. Methanol (MeOH) was used as a solvent instead of water, and a dielectric layer of hafnium dioxide (HfO2) was implemented over the Ti/Au electrode to suppress the dominant Faradaic reaction manifesting as water electrolysis. Qualitative control experiments were performed using parallel electrode pairs and electrically neutral dye (Rhodamine-B) to ascertain impacts on spatiotemporal fluorescent emission intensity from Faradaic reactions as well as convective flow. Spatial analyses were performed inspecting ion concentration alteration under each region of interest within the system. Finally, ion concentration gradients were quantified as a function of applied potential from 5 to 10 Vpp (peak-to-peak potential) and frequencies from 5- to 25-times the electrode charging frequency.
Results and Discussion
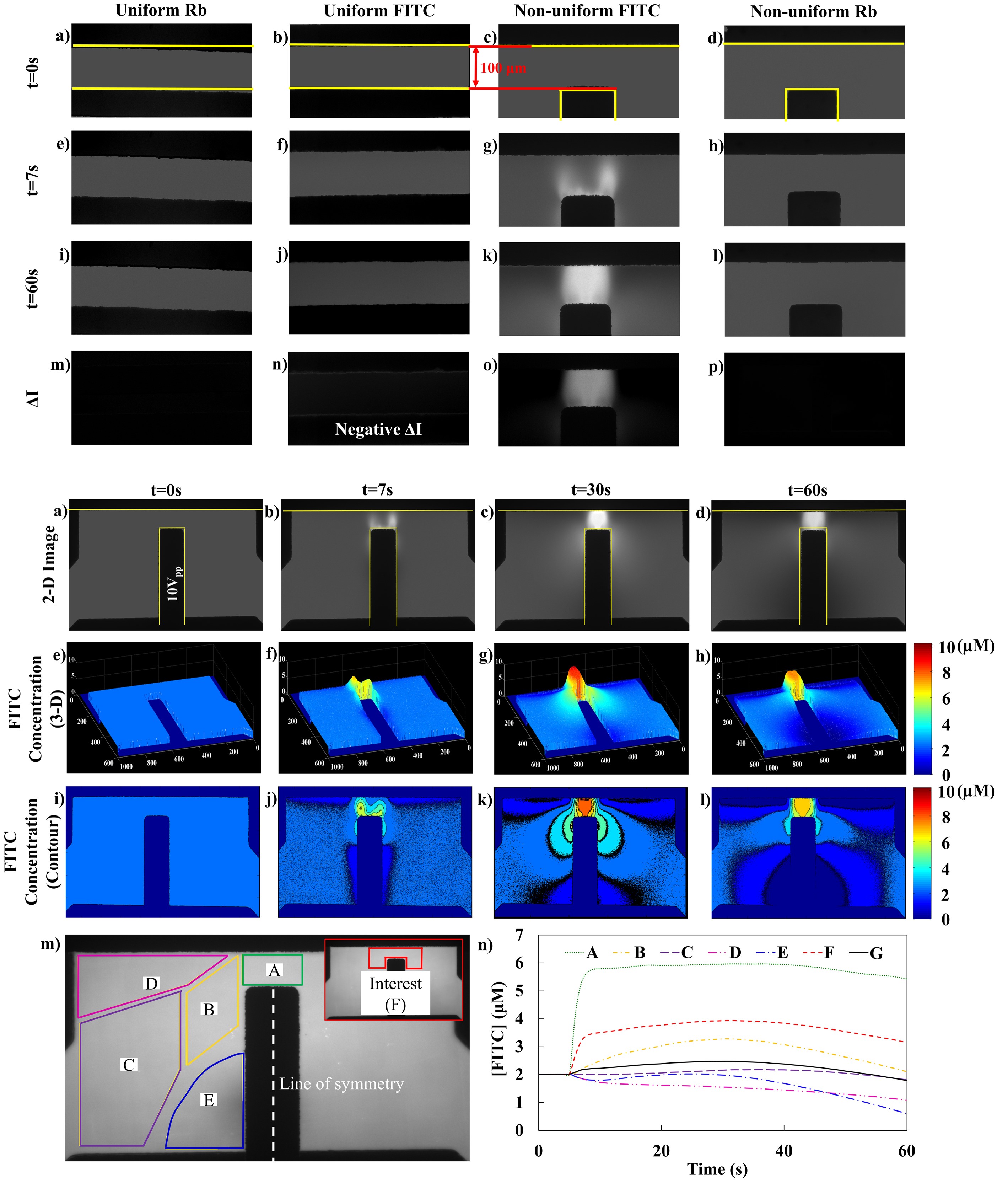
Control experiments were designed and conducted guided by the Nernst-Plank equation. Effect of Faradaic reaction on concentration change was inspected using FITC dye in parallel electrode pair (Fig. 1, 2nd column). Effect of convection flow on concentration change was inspected using Rb dye in non-uniform electrode pair within ionic solutions that had similar conductivity with the FITC solution. Under 10 Vpp and 100 Hz sinusoidal signals, generation of concentration gradient was specifically observed by FITC in non-uniform electric field, but not FITC in uniform electric field, nor Rb in non-uniform electric field. These results demonstrate that the concentration gradient was generated solely by electromigration, but not Faradaic reaction nor convection flow.
Additional temporal and spatial analyses demonstrate FITC concentration increased in the higher electric field density areas (Fig. 2, Areas A, B, F), while decreased in the lower electric field density areas (Fig. 2, Areas C, D, E), at longer term of 60 s (over 6,000 AC cycles). Noticeably, area G (the entire imaged area) illustrates conservation of FITC within the entire observed system (Fig. 2).
Conclusions
Here, we present, for the first time, the feasibility for a reaction-free approach to generate spatiotemporally controllable gradient leveraging biased electromigration induced by spatially non-uniform AC electric fields. Future work will be focused on adapting this approach for generation of physiologically relevant gradients for biological research.
Figure 1: Control experiments under 100 Hz, 10 Vpp. Each row represents test results at t=0 (row 1), 7 (row 2), 60s (row 3), and the net difference between row 3 and row 1. First column demonstrates Rb emission intensity under uniform electric field; Second column demonstrate FITC emission intensity under uniform electric field; Third column demonstrates FITC intensity under non-uniform electric field; and Fourth column demonstrates Rb intensity under non-uniform electric field. Zero intensity change in panel p) indicates the concentration gradient was not driven by convectional flow. The slight negative intensity change in panel n) indicates the concentration gradient was not driven by Faradaic reaction.
Figure. 2: a)-d): Gray-scale 2-D FITC concentration plot obtained from MATLAB image analysis at t=0, 7, 30 and 60s under 10Vpp 100 Hz; e)-h): 3-D MATLAB plot at same time point and i)-l): Matlab contour plot showing the FITC concentration gradient. Boxes A-F in figure m) shows the positions of sampled intensity. Curves A-F in n) demonstrate the time dependency of the averaged FITC concentration within each corresponding boxed areas A-F. Curve G demonstrate the averaged intensity within the entire imaged area.