(558c) Modulating Pegylation of Cationic Poly(amido amine) Dendrimers to Control Electrostatic-Based Drug Delivery to Articular Cartilage
AIChE Annual Meeting
2022
2022 Annual Meeting
Materials Engineering and Sciences Division
Biomaterials for Drug Delivery I: Particle Platforms
Wednesday, November 16, 2022 - 4:04pm to 4:21pm
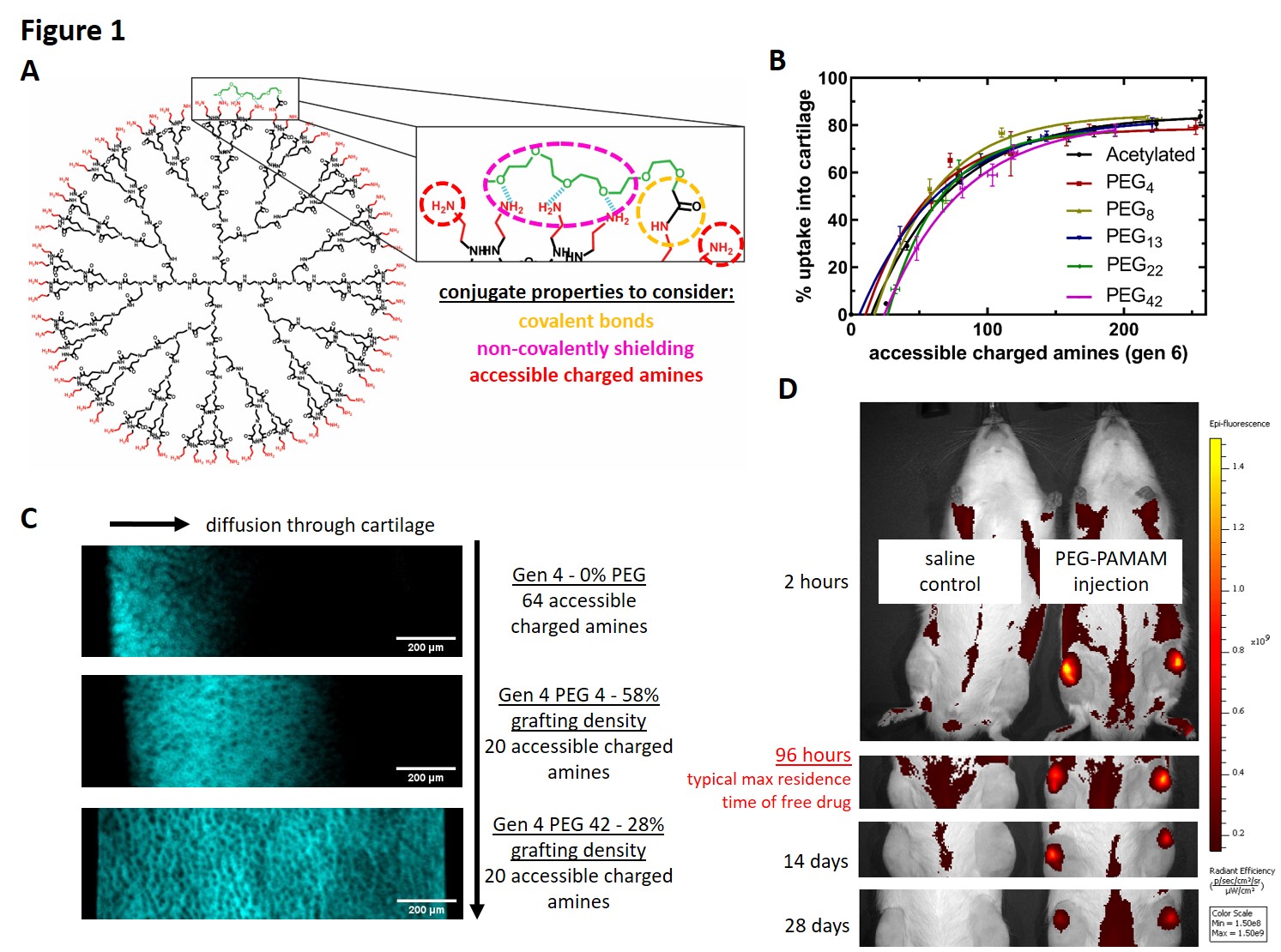
Previously, positively charged, multivalent carriers of size less than 15 nm have been shown to leverage electrostatic interactions to bind to and penetrate through articular cartilage faster than they can be cleared from the joint space. Among those studied are poly(amido amine) dendrimers (PAMAM) which consist of a hierarchically branched polymer with a high surface density of cationic primary amines, ideal for electrostatic-based drug delivery and carrier covalent modification. The cytotoxicity of the polymer’s high charge density can be mitigated by covalent conjugation of poly(ethylene glycol) (PEG) to its surface. Though literature shows that surface-bound PEG oligomers are able to enhance tissue penetration of bound protein while reducing uptake and cytotoxicity of the dendrimer, the role that the PEG corona plays in these interactions has not been explored. Here, a novel salt-based method was used to characterize the PEG corona and number of PAMAM primary amines accessible to the physiological environment (Figure 1A). We tested a library of PEG-PAMAM conjugates consisting of two dendrimer generations, five PEG chain lengths, and a wide range of PEG grafting densities.
When tested in an ex vivo bovine cartilage model, the dendrimer-cartilage interactions were found to be solely dependent on the PEG chain length and the number of accessible charged amines. Cartilage uptake (Figure 1B) and chondrocyte cytotoxicity both increase with increasing accessible charged amines, independent of PEG chain length. The kinetics of tissue uptake is enhanced by greater accessible charged amines and shorter PEG chain lengths, whereas diffusion through cartilage is hindered (Figure 1C). From these observations, it is determined that cartilage uptake and diffusion are dictated by the strength and reversibility of dendrimer-cartilage interactions. PEG-PAMAM conjugates with a large number of accessible charged amines have high cartilage binding affinities, enhancing cartilage uptake but hindering diffusion. Longer PEG chains disrupt the electrostatic interactions between dendrimer and cartilage, introducing reversibility to the dendrimer-cartilage binding, facilitating greater diffusion of conjugates through cartilage but reduced uptake. Formulations with substantial cartilage diffusion were tested in vivo through intra-articular injections into healthy rat joints. Preliminary pharmacokinetic data indicate that conjugates with a greater number of accessible charged amines exhibited prolonged retention, increasing rat joint residence times from three days for free drug, to over 30 days (Figure 1D). These data suggest that, in order to overcome barriers associated with local delivery of osteoarthritis therapies, sufficient cartilage binding strength is necessary for rapid uptake into cartilage. However, reversibility of electrostatic binding is necessary for diffusion throughout cartilage.
With a greater understanding of how the PEG corona influences PEG-PAMAM’s electrostatic-based drug delivery to cartilage, researchers can tune the drug delivery properties to overcome local delivery barriers and fit desired delivery profiles. For biologic delivery, the most prolonged joint residence time may be optimal for sustained delivery of dendrimer-bound protein, whereas specific diffusion profiles or uptake kinetics might be optimal for small molecule therapies that must be cleaved from the dendrimer. Furthermore, these findings may be translatable to drug delivery to other avascular tissues, such as intervertebral discs or cornea.