(353f) Performance of Cyclic Hydrogenation and Dehydrogenation of Benzyltoluene As Liquid Organic Hydrogen Carrier (LOHC)
AIChE Annual Meeting
2022
2022 Annual Meeting
Topical Conference: Sustainable Pathways Toward Hydrogen and Synthetic Fuels
Sustainable Pathways to Clean Hydrogen and Synthetic Fuels II
Tuesday, November 15, 2022 - 2:35pm to 3:00pm
Transformation of the global energy system requires green hydrogen technologies since they enable energy storage in large quantities and over long periods of time as well as global energy distribution. For such large-scale hydrogen storage and transportation, many technologies have been discussed. The LOHC technology offers a unique selling point, as the hydrogen is reversibly chemically bound in a liquid hydrocarbon carrier by catalytic hydrogenation and dehydrogenation reactions. These hydrocarbons enable utilisation of existing infrastructure for liquid fuels for the hydrogen logistics.1,2 To date, two hydrocarbon-based heteroatom-free LOHC systems have been intensively studied in the literature, namely toluene/ methylcyclohexane3,4 and dibenzyltoluene/perhydro dibenzyltoluene.5-7 Despite the fact, that the benzyltoluene/perhydro benzyltoluene system was already mentioned in 2014,8 this alternative system has been studied far less. Only recently, two studies examined the H0‑BT/H12‑BT LOHC system in greater detail.9,10 It was concluded that H0‑BT/H12‑BT is particularly interesting as it combines fast production of high purity hydrogen, which is typically associated to dibenzyltoluene-based LOHC systems, with the technically relevant low viscosity and fast pore diffusion characteristics during dehydrogenation, which is typically associated with methylcyclohexane. Motivated thereby, we conduct a more detailed study of the H0‑BT/H12‑BT LOHC system with analysis of the rate of hydrogen storage and hydrogen release while assessing the stability of this LOHC system in repeated storage cycles.
Experimental
Semi continuous experiments for studying the hydrogenation/dehydrogenation cycles were carried out in a stainless-steel high pressure/high temperature autoclave (Type 4575A, Parr Instruments). The general procedure for performing the cycles was adopted from a previous study focussing on catalyst testing and LOHC stability.5,11 Hydrogenation reactions were performed in a dead-end mode and a pressure of typically 30 barabs was maintained in the reactor by a pressure regulator. The dehydrogenation reactions were initiated by stopping hydrogen supply and opening the reactor to a back pressure regulator. For dehydrogenations, the overall pressure was set to ensure a hydrogen partial pressure of 1.6 barH2. The temperature was controlled by an electric heating mantle and an immersed cooling coil. The utilized LOHC of technical quality was applied without further preconditioning or purification and the finely ground EleMax-102D (Cl#102, 0.3 wt.-% Pt/Al2O3, Clariant) served as catalyst.
The degree of hydrogenation (DoH) and the by‑product formation were determined by liquid sample analysis by means of gas chromatography (GC). The GC was equipped with mass-spectrometry (MS) and flame-ionisation (FID) detectors to identify and quantify the LOHC species. The DoH of the sample is calculated from GC peak areas using equation (1).
Results and Discussion
Hydrogenation of H0-BT and dehydrogenation of H12-BT in repeated cycles
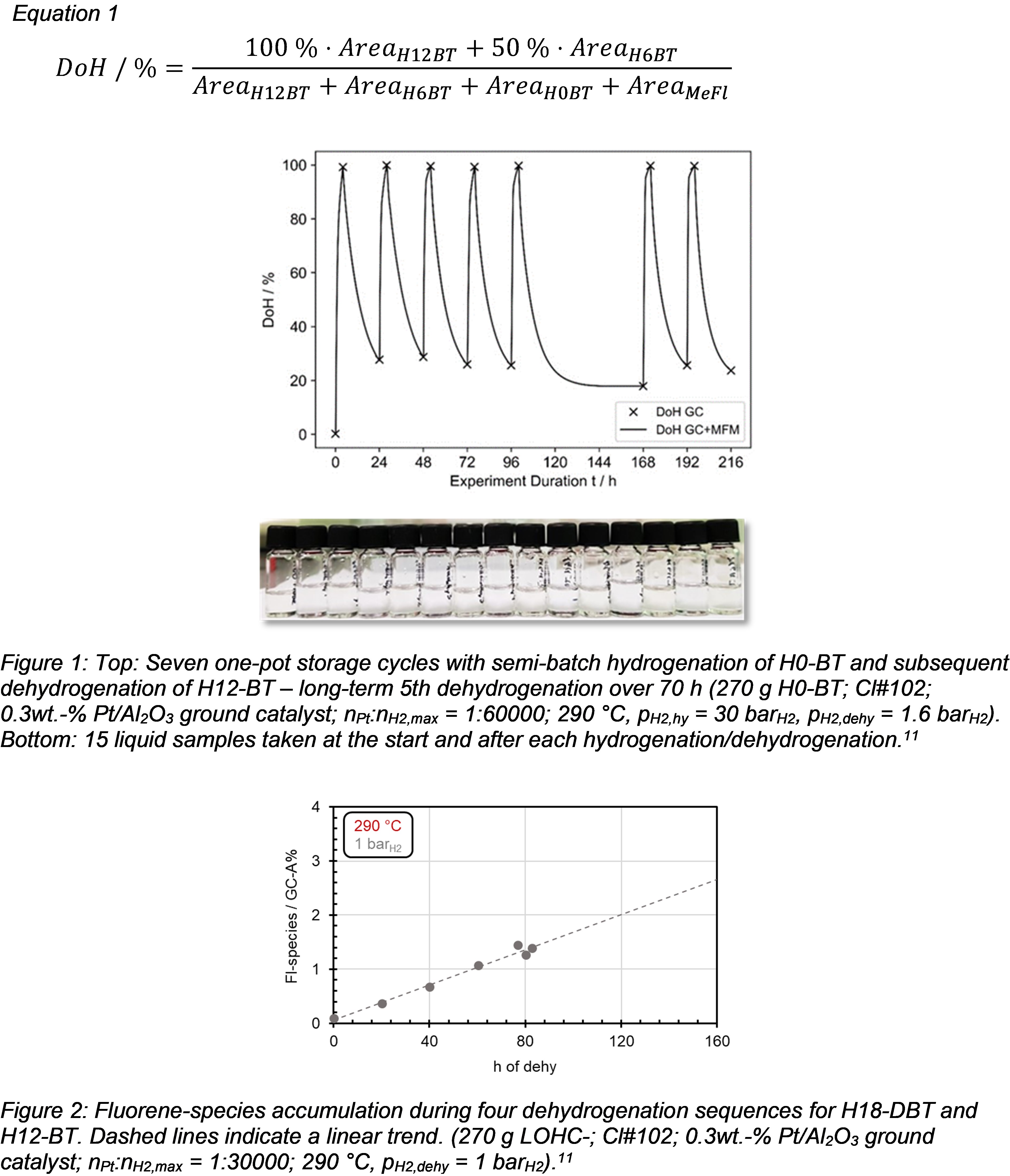
Here, we demonstrate up to seven repeated storage cycles at 290 °C. Hydrogenation was completed after 4 h with a mean DoH of (99.6 ± 0.2)%. In the subsequent dehydrogenation sequences over 20 h, DoHs of 26.2% were reached as consequence of the relatively elevated hydrogen partial pressure. The prolonged 5th dehydrogenation resulted in a DoH of 17.9%, which represents the chemical equilibrium under the applied conditions.11 In another experimental series, the H0‑BT hydrogenations were repeatedly demonstrated to be 260% more productive than the H0‑DBT hydrogenations. The H12‑BT dehydrogenations were 30% more productive than H18‑DBT dehydrogenations, with the latter showing a decreased hydrogen storage capacity over the cycles, whereas the H12‑BT dehydrogenation sequences showed remarkably stable productivities up to a total operation time of 216 h.11
Comparing these results from Figure 1 with literature reports on storage cycles with H0‑DBT/H18‑DBT under identical conditions is quite instructive. Jorschick et al. reported significant catalyst deactivation during long-term dehydrogenation.12 In contrast, the herein reported storage cycles with H0‑BT/H12‑BT, the catalyst activity and LOHC stability appeared unaltered. Even the long-term 5th dehydrogenation over 70 h reaching equilibrium conversion did not impair the catalyst, while yielding the same DoH (25.6%) in the 6th as in the 4th dehydrogenation.
Monitoring of fluorene formation during extended storage cycles
Exposure of the hydrogenation/dehydrogenation catalyst to LOHC systems at low hydrogen partial pressures in combination with elevated temperatures may result in catalyst deactivation and high-boiling by‑product formation.12 In our experiments, four high-boiling regioisomeric methylfluorenes were the only significant by-products detected by means of GC-MS and GC-FID. Expectedly, their concentration increased with every storage cycle. However, fluorene derivatives themselves have been considered as promising LOHC compounds in the literature reflecting their ability to store/release six hydrogen molecules resulting in a comparable hydrogen storage capacity as the H0-BT/H12-BT LOHC system.13 Unfortunately, fluorene derivatives can also trigger the formation of even larger condensation products and finally coke. Therefore, we investigated the methylfluorene formation in four storage cycles at 290 °C and 1.0 barH2 during dehydrogenations. By linear regression, an increase of fluorene derivatives with each dehydrogenation of about 0.32%-points can be estimated (Figure 2). The experiments strongly indicate that BT forms less by-products than DBT.11 Aside from this quantitative analysis, the liquid samples of BT experiments (such as those shown in Figure 1) hardly displayed any visible coloration with increasing operation time. This can be seen as strong indication for the formation of only trace amounts of heavy-boiling by‑products, which are distinguishable by a yellow or brownish colouring.
Conclusions
Inspired by the low viscosity of H12-BT, when compared to H18‑DBT, we have collected additional technically relevant data to assess the H0‑BT/H12‑BT system for technical use in the frame of the LOHC technology. In detail, we have studied the stabilities of the LOHC compounds and the performance of a technical Pt-based hydrogenation/dehydrogenation catalyst over repeated storage cycles. We found that the hydrogen storage cycles under benign conditions (290 °C with 30 bar hydrogen pressure in loading and 1.0 – 1.6 barH2 hydrogen pressure in release sequences) showed an excellent reproducibility. Seven storage cycles with one long-term dehydrogenation over several days neither showed signs for degradation of the catalyst nor of the LOHC compounds. We anticipate that the H0‑BT/H12‑BT LOHC system may represent the preferred hydrocarbon-based and heteroatom-free LOHC system for further technical application with clear advantages over the more traditional toluene/methylcyclohexane and H0‑DBT/H18‑DBT LOHC systems.
References
1. Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K., Liquid organic hydrogen carriers (LOHCs) – techno-economic analysis of LOHCs in a defined process chain. Energy & Environmental Science 2019, 12 (1), 290-307.
2. Niermann, M.; Timmerberg, S.; Drünert, S.; Kaltschmitt, M., Liquid Organic Hydrogen Carriers and alternatives for international transport of renewable hydrogen. Renewable and Sustainable Energy Reviews 2021, 135, 110171.
3. Ichikawa, M., Organic liquid carriers for hydrogen storage. In Solid-State Hydrogen Storage, Walker, G., Ed. Woodhead Publishing: Sawston, Cambridge, 2008; Vol. 1, pp 500-532.
4. Okada, Y.; Shimura, M. In Development of large-scale H2 storage and transportation technology with Liquid Organic Hydrogen Carrier (LOHC), Technical paper at Joint GCC-JAPAN Environment Symposia, Japan, Japan, 2013.
5. Jorschick, H.; Preuster, P.; Dürr, S.; Seidel, A.; Müller, K.; Bösmann, A.; Wasserscheid, P., Hydrogen storage using a hot pressure swing reactor. Energy & Environmental Science 2017, 10 (7), 1652-1659.
6. Modisha, P.; Bessarabov, D., Stress tolerance assessment of dibenzyltoluene-based liquid organic hydrogen carriers. Sustainable Energy & Fuels 2020, 4 (9), 4662-4670.
7. Müller, K.; Aslam, R.; Fischer, A.; Stark, K.; Wasserscheid, P.; Arlt, W., Experimental assessment of the degree of hydrogen loading for the dibenzyl toluene based LOHC system. International Journal of Hydrogen Energy 2016, 41 (47), 22097-22103.
8. Brückner, N.; Obesser, K.; Bösmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P., Evaluation of Industrially applied heatâ€transfer fluids as liquid organic hydrogen carrier systems. ChemSusChem 2014, 7 (1), 229-235.
9. Jorschick, H.; Geißelbrecht, M.; Eßl, M.; Preuster, P.; Bösmann, A.; Wasserscheid, P., Benzyltoluene/dibenzyltoluene-based mixtures as suitable liquid organic hydrogen carrier systems for low temperature applications. International Journal of Hydrogen Energy 2020, 29 (45), 14897-14906.
10. Kwak, Y.; Kirk, J.; Moon, S.; Ohm, T.; Lee, Y.-J.; Jang, M.; Park, L.-H.; Ahn, C.-i.; Jeong, H.; Sohn, H.; Nam, S. W.; Yoon, C. W.; Jo, Y. S.; Kim, Y., Hydrogen production from homocyclic liquid organic hydrogen carriers (LOHCs): Benchmarking studies and energy-economic analyses. Energy Conversion and Management 2021, 239, 114124.
11. Rüde, T.; Dürr, S.; Preuster, P.; Wolf, M.; Wasserscheid, P., Benzyltoluene/perhydro benzyltoluene – Pushing the performance limits of pure hydrocarbon liquid organic hydrogen carrier (LOHC) systems. Sustainable Energy & Fuels 2022, 6, 1541-1553.
12. Jorschick, H.; Dürr, S.; Preuster, P.; Bösmann, A.; Wasserscheid, P., Operational Stability of a LOHC-Based Hot Pressure Swing Reactor for Hydrogen Storage. Energy Technology 2019, 7 (1), 146-152.
13. Sotoodeh, F.; Huber, B. J.; Smith, K. J., The effect of the N atom on the dehydrogenation of heterocycles used for hydrogen storage. Applied Catalysis A: General 2012, 419, 67-72.