(28e) Light-Activated Skin Sealants for Rapid Wound Edge Approximation
AIChE Annual Meeting
2022
2022 Annual Meeting
Materials Engineering and Sciences Division
Biomaterials I: Biomaterials for Infection, Wound, and/or Disease Treatment
Sunday, November 13, 2022 - 5:00pm to 5:18pm
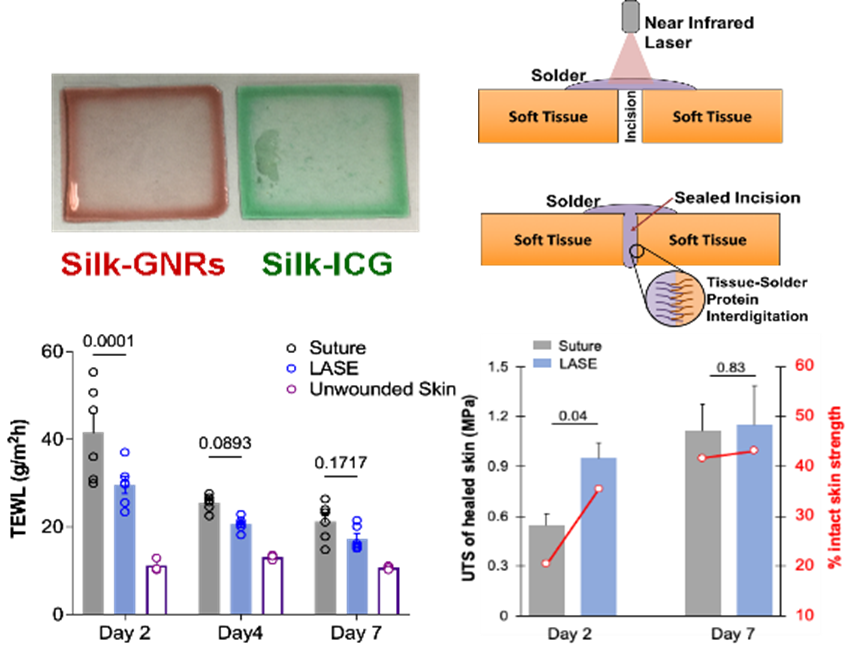
Laser-activated tissue sealing is an alternative approach that employs light-absorbing chromophores for converting near-infrared (NIR) laser light to heat, resulting in the molecular interdigitation of proteins in the tissue and sealant resulting in tissue sealing. Here, we demonstrate a multifunctional technology employing light-activated biomaterials for soft tissue sealing, repair, and combating surgical site infections.
We developed light-activated sealant (LASE) biomaterials consisting of NIR-absorbing chromophores - gold nanorods (GNRs), indocyanine green (ICG) dye (figure 1: top left), copper chloride (CuCl2), or silver nanoprisms (AgNPr) embedded within silk fibroin films. Vancomycin and CuCl2 loaded in the LASE were hypothesized to combat bacterial infections and their efficacy in preventing MRSA growth was evaluated using a modified macrodilution broth method in vitro and skin incision inoculation method in vivo in murine models.
The efficiency of wound healing was determined by measuring i) the ultimate tensile strength (UTS) of the healed skin and ii) transepidermal water loss (TEWL) on day 2 and day 7 post wounding and comparing them with the intact skin and suture-treated controls. TEWL is a measure of the barrier function of the skin and higher values indicate compromised barrier function. TEWL values that are comparable to the control skin provide the measure of the restored barrier function.
Results: Skin incisions closed using every format of LASE resulted in rapid sealing and enhanced or matched the recovery of skin tensile strength and TEWL as compared to sutures as early as 2 days post wounding. Silk-ICG LASE exhibited improved rate of healing in Balb/c as measured by the TEWL and UTS as compared to sutures (figure 1: bottom left and bottom right). Silk-AgNPr LASE exhibited enhanced UTS and comparable TEWL as compared to sutures on day 2 post wounding. Silk-CuCl2 LASE showed UTS values comparable to sutures. However, their inherent antibacterial and angiogenic properties are hypothesized to prevent SSIs and improve the healing outcomes in the long term. In addition, vancomycin-loaded LASE films demonstrated significantly reduced MRSA counts at the wound site when evaluated at seven days post wounding compared to antibacterial sutures. Incisions treated with silk-ICG LASE exhibited equivalent tissue recovery by 7 days and effected coordinated neutrophil (Ly6G+ cells) spatial localization towards the LASE material instead of throughout the wound space at day 2 of healing with similar levels of resolution on day 7.
Conclusion: Our results indicate that laser sealing and rapid approximation with LASE, loaded with molecular enhancers of tissue repair, lead to improved healing outcomes. This can have a significant clinical impact on routine surgeries, acute wounds, and trauma management.